Abstract
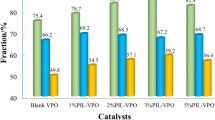
Vanadium oxyphosphate (VPO) with a high surface area was synthesized in a mixed solvent of ionic liquid [Bmim]Br and water, using phosphorous acid to reduce V5+ to V4+ and as a partial phosphorus source, and V2O5 as the vanadium source. The effects of ionic liquids on the synthesis process were investigated in detail. Using pure water as the solvent, the product was a vanadium phosphate compound with large flakes. With the increase in the amount of ionic liquid, the size of the large flakes of vanadium phosphate gradually decreased, and the morphology was changed to spherical particles. When the proportion of ionic liquid was 80% or the ionic liquid was solely used as the solvent, the VPO showed completely spherical particle morphology, and the specific surface area was the highest. X-ray diffraction analysis showed that the crystal phase of VPO changed from vanadium (IV) hydrogenphosphate hemihydrate (VOHPO4·0.5H2O) to an amorphous state with an increase in ionic liquid ratio. All of the above results show that ionic liquids play an important role in the synthesis of VPO materials. After being calcined in an n-butane/air atmosphere, the precursors were transformed into the vanadium pyrophosphate phase. The active VPO catalysts were used in the selective oxidation of cyclohexanol, and the yield of cyclohexanone was 57.98% for the VPO-80 catalyst synthesized using the ionothermal method but only 21.41% for the VPO-W catalyst synthesized using a traditional hydrothermal method, which shows the advantage of ionic liquid synthesis for VPO catalysts.
Graphic Abstract
Vanadium oxyphosphate material with high surface area was synthesized in the mixed solvent of ionic liquid [Bmim]Br and water. Ionic liquid plays an important role in the morphology and properties of synthesized VPO materials. The active VPO catalysts were used in the selective oxidation of cyclohexanol, and the yield of cyclohexanone was 57.98% for VPO-80 catalyst synthesized by ionothermal method, but only 21.41% for VPO-W catalyst synthesized by traditional hydrothermal method, which exhibited the advantages of ionic liquid synthesis for VPO catalysts.









Similar content being viewed by others
References
Lashier ME, Schrader GL (1991) J Catal 128:113–115
Liu J, Wang PC, Feng YN, Xu ZJ, Feng XZ, Ji WJ, Au CT (2019) J Catal 374:171–182
Ivars-Barceló F, Hutchings GJ, Bartley JK, Taylor SH, Sutter P, Amorós P, Sanchis R, Solsona B (2017) J Catal 354:236–249
Hutchings GJ (1991) Appl Catal 72:1–32
Wang F, Dubois JL, Ueda W (2010) Appl Catal A Gen 376:25–32
Li GX, Zhang Q, Fang WG, Zhao Y (2015) Energy Environ Focus 4:301–306
Hutchings GJ (2004) J Mater Chem 14:3385–3395
Cheng MJ, Goddard WA (2013) J Am Chem Soc 135:4600–4603
Dietl N, Wende T, Chen K, Jiang L, Schlangen M, Zhang X, Asmis KR, Schwarz H (2013) J Am Chem Soc 135:3711–3721
Schulz C, Roy SC, Wittich K, Naumann d’Alnoncourt R, Linke S, Strempel VE, Frank B, Glaum R, Rosowski F (2019) Catal Today 333:113–119
He B, Li ZH, Zhang HL, Dai F, Li K, Liu RX, Zhang SJ (2019) Ind Eng Chem Res 58:2857–2867
Leong LK, Chin KS, Taufiq-Yap YH (2012) Appl Catal A Gen 415–416:53–58
Feng XZ, Yao Y, Su Q, Zhao L, Jiang W, Ji WJ, Au CT (2015) Appl Catal B Environ 164:31–39
Hutchings GJ, Kiely CJ, Sananesschulz MT, Burrows A, Volta JC (1998) Catal Today 40:273–286
Feng XZ, Sun B, Yao Y, Su Q, Ji WJ, Au CT (2014) J Catal 314:132–141
Harrouch-Batis N, Batis H, Ghorbel A, Vedrine JC, Volta JC (1991) J Catal 128:248–263
Hutchings GJ, Sananes MT, Sajip S, Kiely CJ, Burrows A, Ellison IJ, Volt JC (1997) Catal Today 33:161–171
Carreon MA, Guliants VV, Pierelli F, Cavani F (2004) Catal Lett 92:11–16
Otaibi RA, Weng WH, Bartley JK, Dummer NF, Kiely CJ, Hutchings GJ (2010) ChemCatChem 2:443–452
Lopez-Sanchez JA, Griesel L, Bartley JK, Wells RPK, Liskowski A, Su DS, Schlögl R, Volta JC, Hutchings GJ (2003) Phys Chem Chem Phys 5:3525–3533
Cooper ER, Andrews CD, Wheatley PS, Webb PB, Wormald P, Morris RE (2004) Nature 430:1012–1016
Liu H, Tian ZJ, Wang L, Wang YS, Li DW, Ma HJ, Xu RS (2016) Inorg Chem 55:1809–1815
Liu L, Yang J, Li JP, Dong JX, Šišak D, Luzzatto M, McCusker LB (2011) Angew Chem Int Ed 50:8139–8142
Parnham ER, Morris RE (2007) Accounts Chem Res 40:1005–1013
Zhu HG, Huang JF, Pan ZW, Dai S (2006) Chem Mater 18:4473–4477
Yang G, Li LH, Wu C, Humphrey MG, Zhang C (2019) Inorg Chem 58:12582–12589
Wagle DV, Zhao H, Baker GA (2014) Accounts Chem Res 47:2299–2308
Wang GM, Valldor M, Siebeneichler S, Wilk-Kozubek M, Smetana V, Mudring AV (2019) Inorg Chem 58:13203–13212
Tan DM, Wang FX, Pietsch T, Grasser MA, Doert T, Ruck M (2019) ACS Appl Energy Mater 2:5140–5145
Chen D, Liu TT, Wang PY, Zhao JH, Zhang CT, Cheng RL, Li WQ, Ji PX, Pu ZH, Mu SC (2020) ACS Energy Lett 5:2909–2915
Wang GM, Valldor M, Dorn KV, Wilk-Kozubek M, Smetana V, Mudring AV (2019) Chem Mater 31:7329–7339
Xue YX, Chen JL, Shao J, Han LQ, Li WL, Sui CH (2020) Mol Catal 492:111010
Parnham ER, Morris RE (2006) Chem Mater 18:4882–4887
Mao Y, Duan H, Xu B, Zhang L, Hu YS, Zhao CC, Wang ZX, Chen LQ, Yang YS (2012) Energy Environ Sci 5:7950–7955
Li XX, Li CQ, Wang RJ, Chen SN, Zhao SJ, Kong XB (2019) Chem Adhes (China) 41:240–269
Acknowledgements
Financial supports from National Natural Science Foundation of China (Grant No. 21763016) and Industrial Support Program for colleges and universities in Gansu Province (Grant No. 2020C-06) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhang, W., Wang, S. et al. Ionic Liquid-Assisted Synthesis of Vanadium Phosphate Catalysts from Phosphorous Acid for Selective Oxidation Reactions. Catal Lett 151, 2366–2375 (2021). https://doi.org/10.1007/s10562-020-03469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03469-5