Abstract
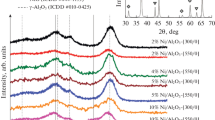
The effect of Ga as support modifier and V as second promoter on the NiMoV/Al2O3-Ga2O3 catalyst varying the synthesis method (SG: sol–gel synthesis vs I: impregnation synthesis) was studied. The catalysts were characterized by elemental analysis, textural properties, XRD, XPS, 27Al NMR, Raman, EDX elemental mapping and HRTEM. The chemical analyses by XRF showed coincidence between experimental and theoretical values according to stoichiometric values proposed to Mo/Ni = 6 and (V + Ni)/(V + Ni + Mo) = 0.35. The sol–gel synthesis method increased the surface area by incorporation of Ga3+ ions into the Al2O3 forming Ga–O–Al bonding; whereas the impregnation synthesis leads to decrease by blocking of alumina pores, as follows NiMoV/Al2O3-Ga2O3(I) < NiMoV/Al2O3-Ga2O3(SG) < Al2O3-Ga2O3(I) < NiMo/Al2O3 < Al2O3-Ga2O3(SG) < Al2O3. The values of BJH mesopores mean size between 6.13 and 7.68 nm. XRD and XPS confirmed a bulk structure typical of (NH4)4[NiMo6O24H6]·5H2O and the presence at the surface of Mo4+, Mo6+, NixSy, NiMoS, Ni2+, Ga3+ and V5+ species, respectively. Raman showed that the sol–gel synthesis method reduces the interactions Ni-Mo sulfide-support and improvement the sulfidation degree NiMoV/Al2O3-Ga2O3(SG) as showed sulfur analysis CHONS. The largest proportion of AlO4 content using the impregnation method to add Ga was verified by 27Al solid-state MAS NMR. The EDS elemental mapping confirmed that Ni, Mo, Al, Ga, V and S are well-distributed on support. The HRTEM analysis shows that the length and stacking distribution of MoS2 crystallites varied from 5.67 to 6.01 nm and 2.46 to 2.74 when using the sol–gel and impregnation synthesis method, respectively. The catalytic results revealed that the synthesis method induced the presence of gallium on the surface influencing the dispersion V5+ species, whose effect could have some relation with strength and density of acid sites that in turn influence the dispersion of the MoS2 phase, which correlates well with the indole HDN activities. The activities as indole HDN pseudo-first-order rate constants’ values (kHDN) from 0.29 to 2.78 mol/(m2·h): NiMoV/Al2O3 < NiMoV/Al2O3-Ga2O3(I) < NiMo/Al2O3 < NiMoV/Al2O3-Ga2O3(SG). Nevertheless, the nature of the active site can be related with reaction pathways, that is, NiMoV/Al2O3-Ga2O3(SG) and NiMoV/Al2O3-Ga2O3(I) catalysts produce ECH through HIND, while NiMoV/Al2O3 and NiMo/Al2O3 produce EB by hydrogenolysis of HIND to OEA. In the regard, the Ga and V act as structural promoters in the NiMo catalysts, allowing the largest generation of NiMoS M-edge-like and BRIM sites for HDN.
Graphic Abstract
The synthesis method influences the reaction pathways for indole HDN, suggesting that NiMoV/Al2O3-Ga2O3(SG) and NiMoV/Al2O3-Ga2O3(I) had the highest production of OEA suggesting that ECH derive from HIND, whereas NiMo/Al2O3 shows that OEA concentration is very low at short times, indicating that ECH derives of OEA










Similar content being viewed by others
References
Gary JH, Handwerk GE (2001) Petroleum Refining Technology and Economics 4a ed. Marcel Dekker, New York
Ledesma BC, Anunziata OA, Beltramone AR (2016) HDN of indolee over Ir-modified Ti-SBA-15. Appl. Catal. B Environ. 192:220–233
Babich IV, Moulijn JA (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 82:607–631
Ministerio de Ambiente y Desarrollo Sostenible, y Ministerio de Minas y Energías, resolución 90963 de 2014 y 40619 de 2017. 2014, pp. 1–3 y 5.
Le Z, Afanasiev P, Li D, Long X, Vrinat M (2008) Solution synthesis of the unsupported Ni–W sulfide hydrotreating catalysts», New Dev. Sulfide Catal. Link. Ind. Needs Fundam. Chall. 130:24–31
Dorbon M, Bernasconi C (1989) Nitrogen compounds in light cycle oils: identification and consequences of ageing. Fuel. https://doi.org/10.1016/0016-2361(89)90077-X
Furimsky E (2003) Metal carbides and nitrides as potential catalysts for hydroprocessing. Appl Catal Gen. https://doi.org/10.1016/S0926-860X(02)00428-3
Kim SC, Massoth FE (2000) Kinetics of the Hydrodenitrogenation of Indolee. Ind. Eng. Chem. Res. 39:1705–1712
Nguyen M-T, Pirngruber GD, Chainet F, Tayakout-Fayolle M, Geantet C (2017) Indolee Hydrodenitrogenation over Alumina and Silica–Alumina-Supported Sulfide Catalysts—Comparison with Quinoline. Ind Eng Chem Res 56:11088–11099. https://doi.org/10.1021/acs.iecr.7b02993
Chianelli RR (1984) Fundamental Studies of Transition Metal Sulfide Hydrodesulfurization Catalysts. Catal Rev 26:361–393. https://doi.org/10.1080/01614948408064718
Debecker DP, Stoyanova M, Rodemerck U, Gaigneaux EM (2011) Preparation of MoO3/SiO2–Al2O3 metathesis catalysts via wet impregnation with different Mo precursors. J Mol Catal Chem 340:65–76. https://doi.org/10.1016/j.molcata.2011.03.011
Breysse M, Afanasiev P, Geantet C, Vrinat M (2003) Overview of support effects in hydrotreating catalysts. Eff Support Hydrotreating Catal Ultra Clean Fuels. https://doi.org/10.1016/S0920-5861(03)00400-0
Cabello CI, Botto IL, Thomas HJ (2000) Anderson type heteropolyoxomolybdates in catalysis. Appl Catal Gen. https://doi.org/10.1016/S0926-860X(99)00535-9
Muralidhar G, Massoth FE, Shabtai J (1984) Catalytic functionalities of supported sulphides: I. Effect of support and additives on the CoMo catalyst. J Catal. https://doi.org/10.1016/0021-9517(84)90108-8
Palcheva R, Kaluža L, Spojakina A, Jirátová K, Tyuliev G (2012) NiMo/γ-Al2O3 Catalysts from Ni Heteropolyoxomolybdate and Effect of Alumina Modification by B Co, or Ni. Chin J Catal. https://doi.org/10.1016/S1872-2067(11)60376-8
Solís-Casados DA, Rodríguez-Nava CE, Klimova T, Escobar-Alarcón L (2020) Selective HDS of DBT using a K2O-modified CoMoW/Al2O3-MgO catalytic formulation. Catal Today. https://doi.org/10.1016/j.cattod.2019.07.029
Escobar-Alarcón L, Klimova T, Escobar-Aguilar J, Romero S, Morales-Ramírez C, Solís-Casados D (2013) Preparation and characterization of Al2O3–MgO catalytic supports modified with lithium. Fuel. https://doi.org/10.1016/j.fuel.2012.10.013
Gutiérrez OY, Klimova T (2011) Effect of the support on the high activity of the (Ni)Mo/ZrO2–SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4,6-DMDBT. J Catal. https://doi.org/10.1016/j.jcat.2011.04.001
Jirátová K, Kraus M (1986) Effect of support properties on the catalytic activity of HDS catalysts. Appl Catal 27:21–29. https://doi.org/10.1016/S0166-9834(00)81043-X
Cimino A, Lo Jacono M, Schiavello M (1975) Effect of zinc, gallium, and germanium ions on the structural and magnetic properties of nickel ions supported on alumina. J Phys Chem. https://doi.org/10.1021/j100570a010
Strohmeier B (1984) Surface spectroscopic characterization of the interaction between zinc ions and $gamma;-alumina. J Catal 86:266–279. https://doi.org/10.1016/0021-9517(84)90372-5
Saini AR, Johnson BG, Massoth FE (1988) Studies of molybdena—alumina catalysts XIV Effect of Cation-Modified Aluminas. Appl Catal. https://doi.org/10.1016/S0166-9834(00)80434-0
Jang JG, Lee YK (2019) Promotional effect of Ga for Ni2P catalyst on hydrodesulfurization of 4,6-DMDBT. Appl Catal B Environ 250:181–188
Zhou W et al (2019) Hydrodesulfurization of 4,6-dimethyldibenzothiophene over NiMo supported on Ga-modified Y zeolites catalysts. J Catal 374:345–359
Zepeda TA, Pawelec B, Díaz de León JN, JA. de los Reyes, A. Olivas (2012) Effect of gallium loading on the hydrodesulfurization activity of unsupported Ga2S3/WS2 catalysts. Appl Catal B Environ 111–112:10–19
Zepeda TA et al (2019) Hydrodesulfurization activity of Ni-containing unsupported Ga(x)WS2 catalysts. Catal. Commun. 130:105760
Altamirano E, J. A. de los Reyes, F. Murrieta, M Vrinat (2008) Hydrodesulfurization of 4,6-dimethyldibenzothiophene over Co(Ni)MoS2 catalysts supported on alumina: Effect of gallium as an additive. Catal. Today 133–135:292–298
Altamirano E, JA de los Reyes, F Murrieta, M Vrinat (2005) Hydrodesulfurization of dibenzothiophene and 4,6-dimethyl-dibenzothiophene: Gallium effect over NiMo/Al2O3 sulfided catalysts. J. Catal. 235:403–412
Díaz JN, de León M, Picquart LM, Vrinat M, JA de los Reyes (2012) Hydrodesulfurization of sulfur refractory compounds: Effect of gallium as an additive in NiWS/γ-Al2O3 catalysts. J Mol Catal Chem 363–364:311–321
Chul Park Y, Rhee HK (1999) Hydrodenitrogenation of pyridine over GaNiMo/Al2O3 catalyst: effect of gallium. Appl Catal Gen 179:145–153
Lo Jacono M, Schiavello M, De Beer VHJ, Minelli G (1977) Effect of gallium ions and of preparation methods on the structural properties of cobalt-molybdenum-alumina catalysts. J Phys Chem 81:1583–1588
Petre AL, Auroux A, Gervasini A, Caldararu M, Ionescu NI (2001) Calorimetric Characterization of Surface Reactivity of Supported Ga2O3 Catalysts. J. Therm. Anal. Calorim. 64:253–260. https://doi.org/10.1023/A:1011557601344
Altamirano E (2005) HDS del DBT y 46 DMDBT sobre catalizadores NiMoCoMo y NiW en estado sulfuro soportados en alúmina: efecto del Ga. DOCTORADO Universidad Autónoma Metropolitana, Mexico
Dejonghe S, Hubaut R, Grimblot J, Bonnelle JP, Des Courieres T, Faure D (1990) Hydrodemetallation of a vanadylporphyrin over sulfided NiMoγAl2O3, MoγAl2O3, and γAl2O3 catalysts—effect of the vanadium deposit on the toluene hydrogenation. Catal Today. https://doi.org/10.1016/0920-5861(90)80009-E
Rankel L, Rollmann L (1983) Catalytic activity of metals in petroleum and their removal. Fuel. https://doi.org/10.1016/0016-2361(83)90250-8
Asaoka S, Nakata S, Shiroto Y, Takeuchi C (1987) Characteristics of Vanadium Complexes in Petroleum Before and After Hydrotreating. In: Filby RH, Branthaver YJF (eds) Metal Complexes in Fossil Fuels, vol 344. American Chemical Society, Washington DC
Lacroix M, Boutarfa N, Guillard C, Vrinat M, Breysse M (1989) Hydrogenating properties of unsupported transition metal sulphides. J Catal 120:473–477
Betancourt P, Marrero S, Pinto-Castilla S (2013) V–Ni–Mo sulfide supported on Al2O3: Preparation, characterization and LCO hydrotreating Fuel Process. Technol 114:21–25
Betancourt P, Rives A, Scott CE, Hubaut R (2000) Hydrotreating on mixed vanadium–nickel sulphides. Catal Today 57:201–207
Méndez FJ, Bastardo-González E, Betancourt P, Paiva L, Brito JL (2013) NiMo/MCM-41 Catalysts for the Hydrotreatment of Polychlorinated Biphenyls. Catal Lett 143:93–100
Haneda M, Kintaichi Y, Mizushima T, Kakuta N, Hamada H (2001) Structure of Ga2O3-Al2O3 prepared by sol–gel method and its catalytic performance for NO reduction by propene in the presence of oxygen. Appl. Catal. B Environ. 31:81–92
Haneda M, Kintaichi Y, Shimada H, Hamada H (2000) Selective Reduction of NO with Propene over Ga2O3–Al2O3: Effect of Sol-Gel Method on the Catalytic Performance. J. Catal. 192:137–148
Puello-Polo E, Marquez E, Brito JL (2018) One-pot synthesis of Nb-modified Al2O3 support for NiMo hydrodesulfurization catalysts. J Sol-Gel Sci Technol 88:90–99
Barrett EP, Joyner LG, Halenda PP (1951) The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 73:373–380
International Centre for Diffraction Data 1995 International Centre for Diffraction Data® (ICDD®), Power Diffraction File, ICDD, Newtown Square Philadelphia
Farojr A, Dossantos A (2006) Cumene hydrocracking and thiophene HDS on niobia-supported Ni, Mo and Ni–Mo catalysts. Catal Today 118:402–409
Li M, Li H, Jiang F, Chu Y, Nie H (2010) The relation between morphology of (Co)MoS2 phases and selective hydrodesulfurization for CoMo catalysts. Catal. Today 149:35–39. https://doi.org/10.1016/j.cattod.2009.03.017
Hensen EJM et al (2001) The Relation between Morphology and Hydrotreating Activity for Supported MoS2 Particles. J. Catal. 199:224–235. https://doi.org/10.1006/jcat.2000.3158
Kasztelan S, Toulhoat H, Grimblot J, Bonnelle JP (1984) A geometrical model of the active phase of hydrotreating catalysts. Appl. Catal. 13:127–159. https://doi.org/10.1016/S0166-9834(00)83333-3
Cid R, Pecchi G (1985) Potentiometric method for determining the number and relative strength of acid sites in colored catalysts Appl. Catal. https://doi.org/10.1016/S0166-9834(00)84340-7
Pizzio LR, Blanco MN (2003) Isoamyl acetate production catalyzed by H3PW12O40 on their partially substituted Cs or K salts. Appl. Catal. Gen. 255:265–277. https://doi.org/10.1016/S0926-860X(03)00565-9
Dalla Costa BO, Legnoverde MS, Lago C, Decolatti HP, Querini CA (2016) Sulfonic functionalized SBA-15 catalysts in the gas phase glycerol dehydration Thermal stability and catalyst deactivation. Microporous Mesoporous Mater 230:66–75
Froment GF, De Wilde J, Bischoff KB (2011) Chemical reactor analysis and design 3 edn. Wiley, Hoboken NJ
Moulijn JA, Tarfaoui A, Kapteijn F (1991) General aspects of catalyst testing. Today Catal. https://doi.org/10.1016/0920-5861(91)87002-5
Thommes M et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. https://doi.org/10.1515/pac-2014-1117
Sampieri A et al (2010) Formation of heteropolymolybdates during the preparation of Mo and NiMo HDS catalysts supported on SBA-15: Influence on the dispersion of the active phase and on the HDS activity. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2009.10.024
Ueno A, Suzuki H, Kotera Y (1983) Particle-size distribution of nickel dispersed on silica and its effects on hydrogenation of propionaldehyde. J Soc Faraday Trans Phys Chem Condens Phases Chem. https://doi.org/10.1039/f19837900127
Ayala-G M, E. Puello P, P. Quintana, G. González-García, C. Diaz, (2015) Comparison between alumina supported catalytic precursors and their application in thiophene hydrodesulfurization: (NH 4) 4 [NiMo 6 O 24 H 6 ]·5H 2 O/γ-Al 2 O 3 and NiMoOx/γ-Al 2 O 3 conventional systems. RSC Adv 5:102652–102662
Galtayries A, Wisniewski S, Grimblot J (1997) Formation of thin oxide and sulphide films on polycrystalline molybdenum foils: characterization by XPS and surface potential variations. J Electron Spectrosc Relat Phenom 87:31–44
Weber Th, Muijsers JC, van Wolput JHMC, Verhagen CPJ, Niemantsverdriet JW (1996) Basic Reaction Steps in the Sulfidation of Crystalline MoO3 to MoS2, As Studied by X-ray Photoelectron and Infrared Emission Spectroscopy. J Phys Chem 100:14144–14150
Aigler JM et al (1993) ESCA study of “model” allyl-based molybdenum/silica catalysts. J. Phys. Chem. 97:5699–5702. https://doi.org/10.1021/j100123a039
Mozhaev AV, Nikulshin PA, Pimerzin AA, Maslakov KI, Pimerzin AA (2016) Investigation of co-promotion effect in NiCoMoS/Al2O3 catalysts based on Co2Mo10-heteropolyacid and nickel citrate. Catal Today 271:80–90
Ninh TKT, Massin L, Laurenti D, Vrinat M (2011) A new approach in the evaluation of the support effect for NiMo hydrodesulfurization catalysts. Appl Catal Gen 407:29–39
Schön G (1973) Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium and gallium oxide. J. Electron Spectrosc. Relat. Phenom. 2:75–86. https://doi.org/10.1016/0368-2048(73)80049-0
Escaño MCS et al (2019) On the presence of Ga2O sub-oxide in high-pressure water vapor annealed AlGaN surface by combined XPS and first-principles methods. Appl. Surf. Sci. 481:1120–1126. https://doi.org/10.1016/j.apsusc.2019.03.196
Escalante Y et al (2019) MCM-41-supported vanadium catalysts structurally modified with Al or Zr for thiophene hydrodesulfurization. Appl. Petrochem. Res. 9:47–55. https://doi.org/10.1007/s13203-019-0227-z
Fierro JLG, Arrua LA, Lopez Nieto JM, Kremenic G (1988) Surface properties of Co-precipitated VTiO catalysts and their relation to the selectiveoxidation of isobutene. Appl. Catal. 37:323–338. https://doi.org/10.1016/S0166-9834(00)80770-8
Liu Y-M et al (2004) Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane. J Catal 224:417–428. https://doi.org/10.1016/j.jcat.2004.03.010
Lee C, Yan H, Brus LE, Heinz TF, Hone J, Ryu S (2010) Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 4:2695–2700. https://doi.org/10.1021/nn1003937
Zhang X, Qiao X-F, Shi W, Wu J-B, Jiang D-S, Tan P-H (2015) Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer multilayer to bulk material. Chem Soc Rev 44:2757–2785. https://doi.org/10.1039/C4CS00282B
Wang JA, Bokhimi X, Morales A, Novaro O, López T, Gómez R (1999) Aluminum Local Environment and Defects in the Crystalline Structure of Sol−Gel Alumina. Catalyst J Phys Chem B 103:299–303. https://doi.org/10.1021/jp983130r
Malki A, Mekhalif Z, Detriche S, Fonder G, Boumaza A, Djelloul A (2014) Calcination products of gibbsite studied by X-ray diffraction, XPS and solid-state NMR. J Solid State Chem 215:8–15. https://doi.org/10.1016/j.jssc.2014.03.019
Rakmae S et al (2020) Defining nickel phosphides supported on sodium mordenite for hydrodeoxygenation of palm oil. Fuel Process Technol 198:106236. https://doi.org/10.1016/j.fuproc.2019.106236
YuN Pushkar A, Sinitsky OOP, Kharlanov AN, Lunina EV (2000) Structure and Lewis acid properties of gallia–alumina catalysts. Appl Surf Sci 167:69–78. https://doi.org/10.1016/S0169-4332(00)00510-9
Rangarajan S, Mavrikakis M (2017) On the Preferred Active Sites of Promoted MoS 2 for Hydrodesulfurization with Minimal Organonitrogen Inhibition. ACS Catal 7:501–509. https://doi.org/10.1021/acscatal.6b02735
Vázquez-Garrido I, López-Benítez A, Berhault G, Guevara-Lara A (2019) Effect of support on the acidity of NiMo/Al2O3-MgO and NiMo/TiO2-Al2O3 catalysts and on the resulting competitive hydrodesulfurization/hydrodenitrogenation reactions. Fuel 236:55–64. https://doi.org/10.1016/j.fuel.2018.08.053
Sattayanon C, Namuangruk S, Kungwan N, Kunaseth M (2017) Reaction and free-energy pathways of hydrogen activation on partially promoted metal edge of CoMoS and NiMoS: A DFT and thermodynamics study. Fuel Process Technol 166:217–227. https://doi.org/10.1016/j.fuproc.2017.06.003
Ramírez J et al (2020) Interaction of different molecules with the hydrogenation and desulfurization sites of NiMoS supported particles with different morphology. Catal Today 353:99–111. https://doi.org/10.1016/j.cattod.2019.08.032
Acknowledgements
The authors would like to acknowledge financial support to Universidad del Atlántico (through “1º convocatoria interna para apoyo al Desarrollo de trabajos de grado en investigacion formative-nivel pregrado y postgrado”).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Puello-Polo, E., Reales, Y.P., Marquez, E. et al. Effect of Gallium and Vanadium in NiMoV/Al2O3-Ga2O3 Catalysts on Indole Hydrodenitrogenation. Catal Lett 151, 2038–2055 (2021). https://doi.org/10.1007/s10562-020-03438-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03438-y