Abstract
IR operando study of cyclohexene reaction on HY zeolite (Si/Al = 15) was performed to determine the role of the OH groups located in the different zeolitic cages and the silanol groups (SiOH). A parallel between the product yield and type and amount of OH groups poisoned by coking and pyridine pulses was established. The role of silanol and zeolitic OH groups during cyclohexene conversion and the coverage rate of surface groups of the dealuminated HY zeolite, were evaluated by FT-IR operando study. At the beginning of the reaction, zeolite exhibited a very intense deactivation due to coke formation. Coke is initially deposited on strong acid sites, inhibiting the H-transfer activity. Further, coke formation decreased. At this stage of the reaction, pyridine addition markedly decreased the isomerization activity. In parallel, it is noted that mainly SiOH, OH groups at hexagonal prisms, and perturbed OH groups in sodalite cages were mainly affected, indicating that these groups are responsible for the isomerization steps. These results showed that even weakly acidic sites are active sites for isomerization reaction.
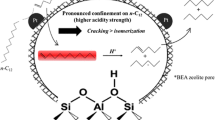
Graphic Abstract
In cyclohexene conversion, hydrogen transfer occurs on supercage OH groups of HY zeolite. In contrast, perturbed sodalite OH and hexagonal prisms OH, as well as SiOH groups, are responsible for isomerization steps, as revealed by pyridine pulse addition during the reaction.








Similar content being viewed by others
References
Du H, Fairbridge C, Yang H, Ring Z (2005) The chemistry of selective ring-opening catalysts. Appl Catal A 294(1):1–21
McVicker G, Daage M, Touvelle M, Hudson CW, Klein DP, Baird M Jr et al (2002) Selective ring opening of naphthenic molecules. J Catal 210(1):137–148
Santana R, Do P, Santikunaporn M, Alvarez W, Taylor J, Sughrue E et al (2006) Evaluation of different reaction strategies for the improvement of cetane number in diesel fuels. Fuel 85(5–6):643–656
Stanislaus A, Marafi A, Rana MS (2010) Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal Today 153(1–2):1–68
Ferraz SGA, Zotin FMZ, Araujo LRR, Zotin JL (2010) Influence of support acidity of NiMoS catalysts in the activity for hydrogenation and hydrocracking of tetralin. Appl Catal A 384(1–2):51–57
Ferraz SGA, Santos BM, Zotin FMZ, Araujo LRR, Zotin JL (2015) Influence of support acidity of NiMo sulfide catalysts for hydrogenation and hydrocracking of tetralin and its reaction intermediates. Ind Eng Chem Res 54(10):2646–2656
Cheng WC, Rajagopalan K (1989) Conversion of cyclohexene over Y-zeolites: a model reaction for hydrogen transfer. J Catal 119(2):354–358
Aboul-Gheit AK, Aboul-Fotouh SM, Aboul-Gheit NAK (2005) Effect of combining palladium, iridium or rhenium with platinum supported on H-ZSM-5 zeolite on their cyclohexene hydroconversion activities. Appl Catal A 292:144–153
Romero-Rivera R, Delvalle M, Alonso G, Flores E, Castillon F, Fuentes S et al (2008) Cyclohexene hydrogenation with molybdenum disulfide catalysts prepared by ex situ decomposition of ammonium thiomolybdate-cetyltrimethylammonium thiomolybdate mixtures. Catal Today 130(2–4):354–360
Vít Z, Gulkova D, Kaluza L, Zdrazil M (2005) Synergetic effects of Pt and Ru added to Mo/AlO sulfide catalyst in simultaneous hydrodesulfurization of thiophene and hydrogenation of cyclohexene. J Catal 232(2):447–455
des Rochettes BM, Marcilly C, Gueguen G, Bousquet J (1990) Kinetic study of hydrogen transfer of olefins under catalytic cracking conditions. Appl Catal 58:35–52
Dwyer J, Karim K, Ojo AF (1991) Bimolecular hydrogen transfer over zeolites and SAPOs having the faujasite structure. J Chem Soc Faraday Trans 87(5):783–796
Li P, Liu X, Zhang C, Chen Y, Huang B, Liu T et al (2016) Selective hydrodesulfurization of gasoline on Co/MoS 2±x catalyst: effect of sulfur defects in MoS 2±x. Appl Catal A 524:66–76
Bukin KA, Potapenko OV, Doronin VP, Sorokina TP, Gulyaeva TI (2017) Performance of a zeolite-containing catalyst and catalysts based on noble metals in intermolecular hydrogen transfer between C6 hydrocarbons. Kinet Catal 58(3):271–278
Boudart M, McConica CM (1989) Catalytic hydrogenation of cyclohexene. J Catal 117:33–41
Soto-Puente M, Del Valle M, Flores-Aquino E, Avalos-Borja M, Fuentes S, Cruz-Reyes J (2007) Synthesis, characterization and cyclohexene hydrogenation activity of high surface area molybdenum disulfide catalysts. Catal Lett 113(3–4):170–175
Alayoglu S, Somorjai GA (2015) Nanocatalysis II: in situ surface probes of nano-catalysts and correlative structure–reactivity studies. Catal Lett 145(1):249–271
Joly JF, Zanier-Szydlowski N, Colin S, Raatz F, Saussey J, Lavalley JC (1991) Infrared in situ characterization of HY zeolite acid sites during cyclohexene transformation. Catal Today 9:31–38
Lesage T, Verrier C, Bazin P, Saussey J, Daturi M (2003) Studying the NOx-trap mechanism over a Pt-Rh/Ba/Al2O3 catalyst by operando FT-IR spectroscopy. Phys Chem Chem Phys 5(20):4435–4440
Jolly S, Saussey J, Lavalley JC, Zanier N, Benazzi E, Joly JF (1993) In situ Ft-Ir study of acid sites responsible for cyclohexene conversion on Hy zeolites. In: Proceedings in 9th international zeolite conference, 1993, pp 319–26
Jolly S, Saussey J, Lavalley LC, Zanier N, Benazzi E, Joly JF (1993) The effect of 2,6-dimethylpyridine poisoning on the activity in n-hexane cracking of dealuminated HY zeolites. Ber Bunsenges Phys Chem 97(3):313–315
Jolly S, Saussey J, Lavalley JC (1994) FT-IR characterization of carbenium ions, intermediates in hydrocarbon reactions on H-ZSM-5 zeolites. Catal Lett 24(1–2):141–146
Jolly S, Saussey J, Lavalley LC (1994) FT-IR study of hydrocarbon conversion on dealuminated HY zeolites in working conditions. J Mol Catal 86:401–421
Li H, Kadam SA, Vimont A, Wormsbecher RF, Travert A (2016) Monomolecular cracking rates of light alkanes over zeolites determined by IR operando spectroscopy. ACS Catal 6(7):4536–4548
Kadam SA, Li H, Wormsbecher RF, Travert A (2018) Impact of zeolite structure on entropic–enthalpic contributions to alkane monomolecular cracking: an IR operando study. Chem Eur J 24:5489–5492
Mendes PSF, Silva JM, Ribeiro MF, Bouchy C, Daudin A (2019) Quantification of the available acid sites in the hydrocracking of nitrogen-containing feedstocks over USY shaped NiMo-catalysts. J Ind Eng Chem 71:167–176
Travert A, Maugé F (1999) IR study of hydrotreating catalysts in working conditions: comparison of the acidity present on the sulfided phase and on the alumina support. In: Delmon B, Froment GF, Grange P (eds) Hydrotreating and hydrocracking of oil fractions. Elsevier, Amsterdam, pp 269–277
Gabrienko AA, Danilova IG, Arzumanov SS, Pirutko LV, Freude D, Stepanov AG (2018) Direct measurement of zeolite brønsted acidity by FTIR spectroscopy: solid-state 1H MAS NMR approach for reliable determination of the integrated molar absorption coefficients. J Phys Chem C 122(44):25386–25395
Khabtou S, Chevreau T, Lavalley JC (1994) Quantitative infrared study of the distinct acidic hydroxyl groups contained in modified Y zeolites. Microporous Mater 3:133–148
Malicki N, Beccat P, Bourges P, Fernandez C, Quoineaud AA, Simon LJ et al (2007) A new model for acid sites in dealuminated Y zeolites. Studies on surface and science catalysis. Elsevier, Amsterdam, pp 762–770
Vimont A, Thibault-Starzyk F, Daturi M (2010) Analysing and understanding the active site by IR spectroscopy. Chem Soc Rev 39(12):4928–4950
Thibault-Starzyk F, Maugé F (2012) Infrared spectroscopy. In: Che M, Védrine JC (eds) Characterization of solid materials and heterogeneous catalysts: from structure to surface reactivity. Wiley, New York
Long J, Wang X, Ding Z, Xie L, Zhang Z, Dong J et al (2008) Cyclopentadiene transformation over H-form zeolites: TPD and IR studies of the formation of a monomeric cyclopentenyl carbenium ion intermediate and its role in acid-catalyzed conversions. J Catal 255:48–58
Gallas JP, Lavalley JC, Burneau A, Barres O (1991) Comparative study of the surface hydroxyl groups of fumed and precipitated silicas 4: infrared study of dehydroxylation by thermal treatments. Langmuir 7(6):1235–1240
Hadjiivanov K (2014) Identification and characterization of surface hydroxyl groups by infrared spectroscopy. Adv Catal 57:99–318
Paweewan B, Barrie PJ, Gladden LF (1999) Coking and deactivation during n-hexane cracking in ultrastable zeolite Y. Appl Catal A 185(2):259–268
West PB, Haller GL, Burwell RL (1973) The catalytic activity of silica gel. J Catal 29(3):486–493
Crépeau G, Montouillout V, Vimont A, Mariey L, Cseri T, Maugé F (2006) Nature, structure and strength of the acidic sites of amorphous silica alumina: an IR and NMR study. J Phys Chem B 110(31):15172–15185
Pine LA, Maher PJ, Wachter WA (1984) Prediction of cracking catalyst behavior by a zeolite unit cell size model. J Catal 85(2):466–476
Liu C, Li G, Hensen EJM, Pidko EA (2015) Nature and catalytic role of extraframework aluminum in faujasite zeolite: a theoretical perspective. ACS Catal 5(11):7024–7033
Maache M, Janin A, Lavalley JC, Joly JF, Benazzi E (1993) Acidity of zeolites Beta dealuminated by acid leaching: an FTIR study using different probe molecules (pyridine, carbon monoxide). Zeolites 13:419–426
Acknowledgments
The authors would like to thank Dr. Philippe Bazin for the support in the pyridine adsorption experiments and operando setup development, as well as Dr. Alexandre Vimont and Yoann Levaque. The authors are also grateful to PETROBRAS and LCS for the financial support and the authorization for using the facilities for carrying out the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santos, B.M., Zotin, J.L., Maugé, F. et al. Strong and Weakly Acidic OH Groups of HY Zeolite into the Different Routes of Cyclohexene Reaction: An IR Operando Study. Catal Lett 151, 1566–1577 (2021). https://doi.org/10.1007/s10562-020-03424-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03424-4