Abstract
Nitro-aromatic pollution in industrial waste streams threat wellbeing of water resources. This study investigates the performance of a copper-based nano catalyst to reduce nitro-aromatic compounds in aqueous solution. Anchoring Cu NPs within the nano spaces of a fibrous silicate with high surface area, and simple accessibility of active sites were successfully established by a facile approach to produce a novel nanocatalyst (DFNS/PEI/Cu). DFNS displayed different properties such as dandelion-like shape, high surface area, and simple availability of active sites. Immobilization of the Cu NPs on DFNS nanospheres not only prevented their aggregation, but also considerably improved the availability of the catalytic active sites. The DFNS/PEI/Cu nanocatalyst demonstrated great catalytic activities for the reduction of nitro compounds under green conditions. Our findings show fibrous DFNS and Cu NPs as a helpful platform for the fabrication of noble metal-based affordable nanocatalyst for many catalytic applications.
Graphic Abstract
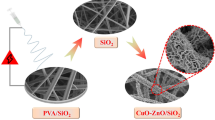
DFNS/PEI/Cu nanocatalyst as a new adsorbents for the reduction of nitro compounds












Similar content being viewed by others
References
Mohammadi M, Rezaei A, Khazaei A, Xuwei S, Huajun Z (2019) ACS Appl Mater Interfaces 11:33194–33206
Ramazani A, Khoobi M, Sadri F, Tarasi R, Shafiee A, Aghahosseini H, Joo SW (2018) Appl Organomet Chem 32:e3908
Mohammadi M, Khazaei A, Rezaei A, Huajun Z, Xuwei S (2019) ACS Sustain Chem Eng 7:5283–5291
Sadeghzadeh SM (2016) J Mol Liq 223:267–273
Rezayati S, Ramazani A (2020) Res Chem Intermed 46:3757–3799
Taghavi Fardood S, Moradnia F, Ramazani A (2019) Micro Nano Lett 14:986–991
Taghavi Fardood S, Ramazani A, Golfar Z, Joo SW (2017) Appl Organomet Chem 31:e3823
Sadeghzadeh SM, Daneshfar F, Malekzadeh M (2014) Chin J Chem 32:349–355
Aghahosseini H, Ramazani A (2020) Eurasian Chem Commun 2:410–419
Mehr ES, Sorbiun M, Ramazani A, Fardood ST (2018) J Mater Sci Mater Electron 29:1333–1340
Saadati SM, Sadeghzadeh SM (2018) Catal Lett 148:1692–1702
Aghahosseini H, Ramazani A, Ślepokura K, Lis T (2018) J Colloid Interface Sci 511:222–232
Fattahi N, Triantafyllidis K, Luque R, Ramazani A (2019) Catalysts 9:758
Motevalizadeh SF, Alipour M, Ashori F, Samzadeh-Kermani A, Hamadi H, Ganjali MR, Aghahosseini H, Ramazani A, Khoobi M, Gholibegloo E (2018) Appl Organomet Chem 32:e4123
Sadeghzadeh SM, Zhiani R, Emrani S (2018) Catal Lett 148:119–124
Tarasi R, Ramazani A, Ghorbanloo M, Khoobi M, Aghahosseini H, Joo SW, Shafiee A (2018) Silicon 10:257–265
Varnaseri N, Rouhani F, Ramazani A, Morsali A (2020) Dalton Trans 49:3234–3242
Zhang K, Suh JM, Lee TH, Cha JH, Choi JW, Jang HW, Varma RS, Shokouhimehr M (2019) Convergence 6:6
Abdullahi Md Amir MA, Asiri SM, Korkmaz AD, Baykal A, Soylu GSP, Karakuş S, Kilislioğlu A (2018) Catal Lett 148:1130–1141
Zhang K, Suh JM, Choi JW, Jang HW, Shokouhimehr M, Varma RS (2019) ACS Omega 4:483–495
Sagar S, Sengupta S, Mota AJ, Chattopadhyay SK, Ferao AE, Riviere E, Lewis W, Naskar S (2017) Dalton Trans 46:1249–1259
Dhara K, Roy P, Ratha J, Manassero M, Banerjee P (2007) Polyhedron 26:4509–4517
Sommer MG, Rechkemmer Y, Suntrup L, Hohloch S, van der Meer M, van Slageren J, Sarkar B (2016) Dalton Trans 45:17770–17781
Vangdal B, Carranza J, Lloret F, Julve M, Sletten J (2002) J Chem Soc Dalton Trans 566–574
Gennarini F, David R, López I, Le Mest Y, Réglier M, Belle C, Thibon-Pourret A, Jamet H, Le Poul N (2017) Inorg Chem 56:7707–7719
Jang Y, Kim S, Jun SW, Kim BH, Hwang S, Song IK, Kim BM, Hyeon T (2011) Chem Commun 47:3601–3603
Khan FA, Dash J, Sudheer C, Gupta RK (2003) Tetrahedron Lett 44:7783–7787
Rai G, Jeong JM, Lee YS, Kim HW, Lee DS, Chung JK, Lee MC (2005) Tetrahedron Lett 46:3987–3990
Shen Y, Su Y, Ma Y (2015) RSC Adv 5:7597–7603
Figueras F, Coq B (2001) J Mol Catal A 173:223–230
Lagrost C, Preda L, Volanschi E, Hapiot P (2005) J Electroanal Chem 585:1–7
Magdalene RM, Leelamani EG, Nanje GNM (2004) J Mol Catal A 223:17–20
Cardenas-Lizana F, Gomez-Quero S, Keane MA (2008) Catal Commun 9:475–481
Duan Z, Ma G, Zhang W (2012) Bull Korean Chem Soc 33:4003–4006
Yan N, Yuan Y, Dyson PJ (2013) Dalton Trans 42:13294–13304
Paganelli S, Piccolo O, Baldi F, Tassini R, Gallo M, La Sorella G (2013) Appl Catal A 451:144–152
Shiraishi Y, Fujiwara K, Sugano Y, Ichikawa S, Hirai T (2013) ACS Catal 3:312–320
Imamura K, Yoshikawa T, Nakanishi K, Hashimoto K, Kominami H (2013) Chem Commun 49:10911–10913
Lee H, Habas SE, Kweskin S, Butcher D, Somorjai GA, Yang P (2006) Angew Chem Int Ed 45:7824–7828
Zeng J, Zhang Q, Chen J, Xia Y (2009) Nano Lett 10:30–35
Wang M-L, Jiang T-T, Lu Y, Liu H-J, Chen Y (2013) J Mater Chem A 1:5923–5933
Lin Y, Qiao Y, Wang Y, Yan Y, Huang J (2012) J Mater Chem 22:18314–18320
Fang Y, Wang E (2013) Nanoscale 5:1843–1848
Ganapathy D, Sekar G (2013) Catal Commun 39:50–54
Feng G, Liu F, Lin C, Li W, Wang S, Qi C (2013) Catal Commun 37:27–31
Yuan G, Keane MA (2007) Ind Eng Chem Res 46:705–715
Wei S, Ma Z, Wang P, Dong Z, Ma J (2013) J Mol Catal A 370:175–181
Li W, Zhang B, Li X, Zhang H, Zhang Q (2013) Appl Catal A 459:65–72
Polshettiwar V, Cha D, Zhang X, Basset JM (2010) Angew Chem 49:9652–9656
Fihri A, Bouhrara M, Patil U, Cha D, Saih Y, Polshettiwar V (2012) ACS Catal 2:1425–1431
Lilly Thankamony AS, Lion C, Pourpoint F, Singh B, Perez Linde AJ, Carnevale D, Bodenhausen G, Vezin H, Lafon O, Polshettiwar V (2015) Angew Chem 54:2190–2193
Sdeghzadeh SM (2016) Catal Sci Technol 6:1435–1441
Parlett CM, Wilson K, Lee AF (2013) Chem Soc Rev 42:3876–3893
Sadeghzadeh SM, Zhiani R, Emrani S (2017) RSC Adv 7:24885–24894
Sadeghzadeh SM (2015) RSC Adv 5:68947–68952
Zhao Y, Tang JJ, Motavalizadehkakhky A, Kakooeie S, Sadeghzadeh SM (2019) RSC Adv 9:35022–35032
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradi, M., Rastakhiz, N., Ghaedi, M. et al. DFNS/PEI/Cu Nanocatalyst for Reduction of Nitro-aromatic Compounds. Catal Lett 151, 1653–1662 (2021). https://doi.org/10.1007/s10562-020-03422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03422-6