Abstract
Ni–Mo2C and Ni–WC were evaluated in dry reforming of methane employing different CH4/CO2 ratios. Ni–Mo2C remained active under an excess of CH4, but deactivation occurred under an excess of CO2. Ni–WC was resistant to excess of CO2 but showed carbon deposition under excess of CH4.
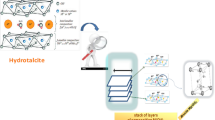
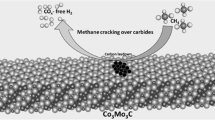
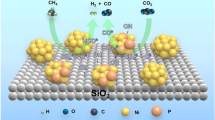
Graphic Abstract











Similar content being viewed by others
References
York APE, Claridge JB, Brungs AJ et al (1997) Molybdenum and tungsten carbides as catalysts for the conversion of methane to synthesis gas using stoichiometric feedstocks. Chem Commun. https://doi.org/10.1039/a605693h
York APE, Claridge JB, Márquez-Alvarez C et al (1997) Synthesis of early transition metal carbides and their application for the reforming of methane to synthesis gas. Stud Surf Sci Catal 110:711–720. https://doi.org/10.1016/S0167-2991(97)81033-6
Claridge JB, York APE, Brungs AJ et al (1998) New catalysts for the conversion of methane to synthesis gas: molybdenum and tungsten carbide. J Catal 180:85–100. https://doi.org/10.1006/jcat.1998.2260
British petroleum (2018) Statistical Review of World Energy 2018
Iyer MV, Norcio LP, Kugler EL, Dadyburjor DB (2003) Kinetic Modeling for Methane Reforming with Carbon Dioxide over a Mixed-Metal Carbide Catalyst. Ind Eng Chem Res 42:2712–2721. https://doi.org/10.1021/ie020677q
UFRJ–IBP (2017) Gás do Pré-Sal Oportunidades Desafios e Perspectivas. In: Colomer M (ed) Ciclo de Debates sobre Petróleo e Economia. Instituto Brasileiro de Petróleo e Gás, Rio de Janeiro, p 46
Rochedo PRR, Costa IVL, Império M et al (2016) Carbon capture potential and costs in Brazil. J Clean Prod 131:280–295. https://doi.org/10.1016/j.jclepro.2016.05.033
Teixeira da Silva VLS (2016) Catalytic Dry Reforming of Methane over Ni/β-Mo2C Catalysts. In: The 11th Natural Gas Conversion Symposium. Tromso, Norway
Hanif A, Suhartanto T, Green MLH (2002) Possible Utilisation of CO2 on Natuna’s Gas Field Using Dry Reforming of Methane to Syngas (CO & H2). In: SPE–Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 8-10. pp 833–840
Usman M, Wan Daud WMA, Abbas HF (2015) Dry reforming of methane: Influence of process parameters—a review. Renew Sustain Energy Rev 45:710–744. https://doi.org/10.1016/j.rser.2015.02.026
Shao H, Kugler EL, Ma W, Dadyburjor DB (2005) Effect of temperature on structure and performance of in-house cobalt-tungsten carbide catalyst for dry reforming of methane. Ind Eng Chem Res 44:4914–4921. https://doi.org/10.1021/ie049186r
Shao H, Kugler EL, Dadyburjor DB et al (2009) Correlating NEXAFS characterization of Co-W and Ni-W bimetallic carbide catalysts with reactivity for dry reforming of methane. Appl Catal A Gen 356:18–22. https://doi.org/10.1016/j.apcata.2008.11.012
Cheng J, Huang W (2010) Effect of cobalt (nickel) content on the catalytic performance of molybdenum carbides in dry-methane reforming. Fuel Process Technol 91:185–193. https://doi.org/10.1016/j.fuproc.2009.09.011
Arora S, Prasad R (2016) An overview on dry reforming of methane: strategies to reduce carbonaceous deactivation of catalysts. RSC Adv 6:108668–108688. https://doi.org/10.1039/C6RA20450C
Yao Z, Jiang J, Zhao Y et al (2016) Insights into the deactivation mechanism of metal carbide catalysts for dry reforming of methane via comparison of nickel-modified molybdenum and tungsten carbides. RSC Adv 6:19944–19951. https://doi.org/10.1039/C5RA24815A
Nikoo MK, Amin NAS (2011) Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process Technol 92:678–691. https://doi.org/10.1016/j.fuproc.2010.11.027
Iyer MV, Norcio LP, Punnoose A et al (2004) Catalysis for synthesis gas formation from reforming of methane. Top Catal 29:197–200. https://doi.org/10.1023/B:TOCA.0000029803.90815.68
de Oliveira PMCR (2016) Carbeto de molibdenio promovido por niquel como catalisador na reforma seca de metano. UFRJ/COPPE, Rio de Janeiro
Li Y, Wang Y, Zhang X, Mi Z (2008) Thermodynamic analysis of autothermal steam and CO2 reforming of methane. Int J Hydrogen Energy 33:2507–2514. https://doi.org/10.1016/j.ijhydene.2008.02.051
Zou H, Chen S, Huang J, Zhao Z (2016) Effect of additives on the properties of nickel molybdenum carbides for the tri-reforming of methane. Int J Hydrogen Energy 41:16842–16850. https://doi.org/10.1016/j.ijhydene.2016.07.108
Bradford MCJ, Vannice MA (1996) Catalytic reforming of methane with carbon dioxide over nickel catalysts II. Reaction kinetics Appl Catal A Gen 142:97–122. https://doi.org/10.1016/0926-860X(96)00066-X
Korobitsyn M, Berkel F Van, Christie G (2000) SOFC as a gas separator–Final Report
Bradford MCJ, Vannice MA (1998) CO2 reforming of CH4 over supported Pt catalysts. J Catal 173:157–171. https://doi.org/10.1006/jcat.1997.1910
Teuner SC, Neumann P, Von Linde F (2001) CO through CO2 reforming –the Calcor standard and Calcor economy processes. Oil Gas Eur Mag 27:44–46
Gunardson H (1998) Industrial gases in petrochemical processing. CRC Press, New York, NY
Maroto-Valer MM, Song C, Soong Y (2002) Environmental challenges and greenhouse gas control for fossil fuel utilization in the 21st century. Kluwer Academic / Plenum Publishers, San Diedo, California
Pienkowski L, Motak M, Dabek R, Jaszczur M (2018) Use of HTGR process heat with catalysts for dry reforming of methane using CO2 to singas for the chemical industry, AGH
Shamsi A (2002) Methane dry reforming over carbide, nickel-based, and noble metal catalysts. In: Song C, Gaffney AF, Fujimoto K (eds) ACS symposium series. American Chemical Society, Washington, DC, pp 182–196
Pakhare D, Spivey J (2014) A review of dry (CO 2) reforming of methane over noble metal catalysts. Chem Soc Rev 43:7813–7837. https://doi.org/10.1039/C3CS60395D
Silva CG, Passos FB, da Silva VT (2019) Influence of the support on the activity of a supported nickel-promoted molybdenum carbide catalyst for dry reforming of methane. J Catal 375:507–518. https://doi.org/10.1016/j.jcat.2019.05.024
Armor JN (1999) The multiple roles for catalysis in the production of H2. Appl Catal A Gen 176:159–176. https://doi.org/10.1016/S0926-860X(98)00244-0
Pritchard ML, McCauley RL, Gallaher BN, Thomson WJ (2004) The effects of sulfur and oxygen on the catalytic activity of molybdenum carbide during dry methane reforming. Appl Catal A Gen 275:213–220. https://doi.org/10.1016/j.apcata.2004.07.035
Gaillard M, Virginie M, Khodakov AY (2017) New molybdenum-based catalysts for dry reforming of methane in presence of sulfur: A promising way for biogas valorization. Catal Today 289:143–150. https://doi.org/10.1016/j.cattod.2016.10.005
Oyama ST (1992) Preparation and catalytic properties of transition metal carbides and nitrides. Catal Today 15:179–200. https://doi.org/10.1016/0920-5861(92)80175-M
Koverga AA, Flórez E, Dorkis L, Rodriguez JA (2020) Promoting effect of tungsten carbide on the catalytic activity of Cu for CO2 reduction. Phys Chem Chem Phys 22:13666–13679. https://doi.org/10.1039/D0CP00358A
Prats H, Gutiérrez RA, Piñero JJ et al (2019) Room temperature methane capture and activation by Ni clusters supported on TiC(001): effects of metal-carbide interactions on the cleavage of the C–H Bond. J Am Chem Soc 141:5303–5313. https://doi.org/10.1021/jacs.8b13552
Hall DS, Lockwood DJ, Bock C et al (2015) Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proceedings Math Phys Eng Sci. https://doi.org/10.1098/rspa.2014.0792
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Resini C, Montanari T, Barattini L et al (2009) Hydrogen production by ethanol steam reforming over Ni catalysts derived from hydrotalcite-like precursors: Catalyst characterization, catalytic activity and reaction path. Appl Catal A Gen 355:83–93. https://doi.org/10.1016/j.apcata.2008.11.029
Oliva P, Leonardi J, Laurent JF et al (1982) Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J Power Sources 8:229–255. https://doi.org/10.1016/0378-7753(82)80057-8
Levin D, Soled SL, Ying JY (1996) Crystal structure of an ammonium nickel molybdate prepared by chemical precipitation. Inorg Chem 35:4191–4197. https://doi.org/10.1021/ic951200s
Rives V, Angeles Ulibarri M (1999) Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord Chem Rev 181:61–120. https://doi.org/10.1016/S0010-8545(98)00216-1
Xiao T, Wang H, York APE et al (2002) Preparation of nickel-tungsten bimetallic carbide catalysts. J Catal 209:318–330. https://doi.org/10.1006/jcat.2002.3651
Lee J (1987) Molybdenum carbide catalysts I. Synthesis of unsupported powders. J Catal 106:125–133. https://doi.org/10.1016/0021-9517(87)90218-1
Lobo AO, Martin AA, Antunes EF et al (2005) Caracterização de materiais carbonosos por espectroscopia Raman. Rev Bras Apl Vácuo 24:98–103. https://doi.org/10.17563/rbav.v24i2.99
Shi C, Zhang A, Li X et al (2012) Ni-modified Mo2C catalysts for methane dry reforming. Appl Catal A Gen 431–432:164–170. https://doi.org/10.1016/j.apcata.2012.04.035
LaMont DC, Gilligan AJ, Darujati ARS et al (2003) The effect of Mo2C synthesis and pretreatment on catalytic stability in oxidative reforming environments. Appl Catal A Gen 255:239–253. https://doi.org/10.1016/S0926-860X(03)00567-2
Guo J, Zhang AJ, Zhu AM et al (2010) A carbide catalyst effective for the dry reforming of methane at atmospheric pressure. In: Hu YH (ed) ACS symposium series. American Chemical Society.pp, Washington, DC, pp 181–196
Gao H, Yao Z, Shi Y, Wang S (2018) Improvement of the catalytic stability of molybdenum carbide via encapsulation within carbon nanotubes in dry methane reforming. Catal Sci Technol 8:697–701. https://doi.org/10.1039/c7cy02506h
Acknowledgments
The authors thank for the financial support provided by CNPq, CAPES, and PETROBRAS. We would like to dedicate this work to the memory of Prof. Victor Teixeira da Silva.
Author information
Authors and Affiliations
Corresponding author
Additional information
Victor Luis dos Santos Teixeira da Silva in memoriam
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barbosa, R.D., Baldanza, M.A.S., de Resende, N.S. et al. Nickel–Promoted Molybdenum or Tungsten Carbides as Catalysts in Dry Reforming of Methane: Effects of Variation in CH4/CO2 Molar Ratio. Catal Lett 151, 1578–1591 (2021). https://doi.org/10.1007/s10562-020-03420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03420-8