Abstract
Co3−xMnxO4 is a bimetal oxide with excellent electrochemical activity in alkaline solution, has been regarded as a promising alternative in the field of ion-air batteries and proton exchange membrane fuel cell (PEMFC). Herein, we report a simple solvothermal-calcination method to fabricate Co3−xMnxO4 with tunable external Co3+/Co2+ and Mn3+/Mn2+ ratio. The tunable ratio of element valence in the bimetal results in a higher exposure of active center for oxygen redox reaction (ORR), and thus lead to a better ORR activity, which was confirmed by X-ray photoelectron spectroscopy characterizations and electrochemical measurements. Specially, Co1.8Mn1.2O4 with a Co3+/Co2+ ratio of 2.08 showed an overpotential of 0.37 V at benchmark ORR current density of 3 mA/cm2 in 0.1 M KOH, which is lower than that of pure oxide (Mn3O4 0.53 V and Co3O4 0.56 V). In addition, the as prepared Co1.8Mn1.2O4 exhibited a positive half-wave potential (0.83 V vs RHE) due to their more active sites, promotes charge transfer, adsorption and desorption of oxygen species. This work provides a strategy for the design and fabrication of earth-abundant, low-cost electrocatalysts for PEMFC in practical applications.
Graphic Abstract
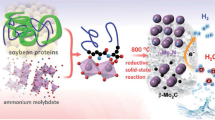
Co3−xMnxO4 was fabricated by tuning external Co3+/Co2+ and Mn3+/Mn2+ ratio, and the activity initially shows a positive correlation with the ration of Co3+/Co2+ in Co3−xMnxO4.










Similar content being viewed by others
References
Tian XL, Lu XF, Xia BY et al (2020) Joule 4:45–68
Shao MH, Chang QW, Dodelet JP et al (2016) Chem Rev 116:3594–3657
Gasteiger HA, Kocha SS, Sompalli B et al (2005) Appl Catal B Environ 56:9–35
Shinozaki K, Zack JW, Richards RM et al (2015) J Electrochem Soc 10:F1144–F1158
Yannick G, Olga A, Kocha E et al (2010) Anal Chem 82:6321–6328
Kocha SS, Shinozaki K, Zack JS et al (2017) Electrocatalysis 8:366–374
Stamenkovic VR, Fowler B, Mun BS et al (2007) Science 315:493–497
Paulus UA, Schmidt TJ, Gasteiger HA et al (2001) J Electroanal Chem 495:134–145
Zhong M, Cano ZP, Yu AP et al (2020) Angew Chem Int Ed 59:18334–18338
Zhong HH, Campos-Roldán CA, Zhao Y et al (2018) Catalysts 8:559
Zheng ZL, Zhang WC, Chen L et al (2019) Appl Surf Sci 483:496–505
Su YC, Liu HC, Li CX et al (2019) J Alloys Compd 799:160–168
Kuznetsov DA, Han BH, Yang Y et al (2018) Joule 2:225–244
Lübke M, Sumboja A, McCafferty L et al (2018) ChemistrySelect 3:2613–2622
Jiang H, Gu JX, Zheng XS et al (2019) Energy Environ Sci 12:322–333
Yang J, Sun HY, Liang HY et al (2016) Adv Mater 28:4606–4613
Tang C, Zhang Q (2017) Adv Mater 29:1604103
Zhu HY, Zhang S, Huang YX et al (2013) Nano Lett 13:2947–2951
Seh ZW, Kibsgaard J, Dickens CF et al (2017) Science 355:eaad4998
Lee DU, Li JD, Park MG et al (2017) ChemSusChem 10:2258–2266
Li C, Han XP, Cheng FY et al (2015) Nat Commun 6:7345
Giordano N, Passalacqua E, Pino L et al (1991) Electrochim Acta 36:1979–1984
Liu J, Jiang LH, Zhang BS et al (2014) ACS Catal 4:2998–3001
Yan DF, Li YX, Huo J et al (2017) Adv Mater 48:1606459
Xiao XL, Liu XF, Zhao H et al (2012) Adv Mater 24:5762–5766
Kreider ME, Stevens MB, Liu YZ et al (2020) Chem Mater 32:2946–2960
Han XP, He GW, He Y et al (2018) Adv Energy Mater 8:1702222
Liao PL, Keith JA, Carter EA (2012) J Am Chem Soc 134:13296–13309
Suntivich J, Hong WT, Lee YL et al (2014) J Phys Chem C 118:1856–1863
Zagal JH, Koper MTM (2016) Angew Chem Int Ed 55:14510–14521
Velmurugan M, Chen SM (2017) Sci Rep 7(1):653
Nolin B, Jones RN (1956) Can J Chem 34:1393–1404
Zou JP, Liu HL, Luo JM et al (2016) ACS Appl Mater Interfaces 8:18140–18149
Chen C, Liu BR, Ru Q et al (2016) J Power Sources 326:252–263
Fu SF, Zhu CZ, Song JH et al (2017) Adv Energy Mater 7:1700363
Gautier JL, Rios E, Gracia M et al (1997) Thin Solid Films 311:51–57
Naveen AN, Selladurai S (2015) RSC Adv 5:65139–65152
Hong WT, Risch M, Stoerzinger KA et al (2015) Energy Environ Sci 8:1404–1427
Qin CL, Ye ZG, Guang M et al (2018) J Electrochem Soc 14:A3496–A3503
Li K, Zhang RG, Gao RJ et al (2019) Appl Catal B Environ 244:536–545
Jiang Y, Deng YP, Fu J et al (2018) Adv Energy Mater 8:1702900
Muraliganth T, Manthiram A (2010) J Phys Chem C 36:15530–15540
Yi D, Lu F, Zhang FC et al (2020) Angew Chem Int Ed 59:15855–15859
Cheng FY, Shen J, Peng B et al (2011) Nat Chem 3:79–84
Wang JK, Gao R, Zhou D et al (2017) ACS Catal 7:6533–6541
Ma TY, Zheng Y, Dai S et al (2014) J Mater Chem A 2:8676–8682
Cao X, Jin C, Lu FL et al (2014) J Electrochem Soc 5:H296–H300
Zhang TW, Li ZF, Wang LK et al (2018) ChemSusChem 11(16):2730–2736
Li MF, Zhao ZP, Cheng T et al (2016) Science 354:1414–1419
Zhou RF, Zheng Y, Jaroniec M et al (2016) ACS Catal 6:4720–4728
Li J, Wang YC, Zhou T et al (2015) J Am Chem Soc 137:14305–14312
Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 21808172), Tianjin Municipal Natural Science Foundation (Nos. 18JCQNJC05800, 18JCZDJC37200), Innovation Fund for Young Talents of TUST and the Fund of Tianjin Key Laboratory of Brine Chemical Engineering and Resource Eco-utilization (No. BCERE201909), Yangtze Scholars and Innovative Research Team in University (IRT-17R81), Innovative Research Team of Tianjin Municipral Education Commission (TD13-5008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10562_2020_3381_MOESM1_ESM.docx
Schematic patterns (Fig. S1), FT-IR images (Fig. S2), SEM images (Fig. S3), XRD patterns (Fig. S4), BET curves (Fig. S5), Full XPS spectrum (Fig. S6 and S7), polarization curves (Fig. S8, S9 and S10), Comparisons of electrocatalytic activity (Table S1), Recent research (Table S2), BET data (Table S3), XPS data (Table S4, S5 and S7). (DOCX 2480 kb)
Rights and permissions
About this article
Cite this article
Zhao, N., Wang, S., Cheng, P. et al. Controllable Fabrication of Co3−xMnxO4 with Tunable External Co3+/Co2+ Ratio for Promoted Oxygen Reduction Reaction. Catal Lett 151, 1810–1820 (2021). https://doi.org/10.1007/s10562-020-03381-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03381-y