Abstract
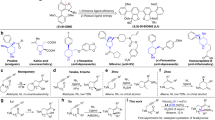
The enantioselective hydroalkoxylation of butadiene with ethanol has been performed in the presence of nickel-based catalysts and chiral diphosphine ligands. Ee’s up to 77% could be obtained from the use of atropoisomeric chiral ligands such as Segphos. The kinetics parameters of the reaction were determined using a qualitative kinetic model to better explain the l/b isomerization and racemization processes observed for long reaction times.
Graphic Abstract











Similar content being viewed by others
References
M. Beller, C. Bolm, Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, Second Revised and Enlarged Edition (Wiley, 2004)
Zeng X (2013) Recent advances in catalytic sequential reactions involving hydroelement addition to carbon–carbon multiple bonds. Chem Rev 113:68646–76900
Adamson NJ, Malcolmson SJ (2020) Catalytic enantio- and regioselective addition of nucleophiles in the intermolecular hydrofunctionalization of 1,3-dienes. ACS Catal 10:1060–1076
Long J, Wang P, Wang W, Li Y, Yin G (2019) Nickel/Brønsted acid-catalyzed chemo- and enantioselective intermolecular hydroamination of conjugated dienes. iScience 22:369–379
Bezzenine-Lafollee S, Gil R, Prim D, Hannedouche J. (2017). First-row late transition metals for catalytic alkene hydrofunctionalisation: recent advances in C-N, C-O and C-P bond formation. Molecules, 22: 1901/1–1901/29.
Greenhalgh MD, Jones AS, Thomas SP (2015) Iron-Catalysed hydrofunctionalisation of alkenes and alkynes. ChemCatChem 7:190–222
Yadav JS, Antony A, Rao TS, Subba R, Basi V (2011) Recent progress in transition metal catalysed hydrofunctionalisation of less activated olefins. J Organomet Chem 696:16–36
Margrey KA, Nicewicz DA (2016) A general approach to catalytic alkene anti-Markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis. Acc Chem Res 49:1997–2006
Liu C, Bender CF, Han X, Widenhoefer RA (2007) Platinum-catalyzed hydrofunctionalization of unactivated alkenes with carbon, nitrogen and oxygen nucleophiles. Chem Commun 35:3607–3618
Obligacion JV, Chirik PJ (2018) Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat Rev Chem 2:15–34
Chen J, Lu Z (2018) Asymmetric hydrofunctionalization of minimally functionalized alkenes via earth abundant transition metal catalysis. Org Chem Front 5:260–272
Ananikov VP, Tanaka MT. (2013). Topics in Organometallic Chemistry 43, Ed Springer.
Hermann N, Vogelsang D, Behr A, Seidensticker T (2018) Homogeneously catalyzed 1,3-diene functionalization—A success story from laboratory to miniplant scale. ChemCatChem 10:5342–4365
Ibrahim AD, Entsminger SW, Zhu L, Fout AR (2016) A highly chemoselective cobalt catalyst for the hydrosilylation of alkenes using tertiary silanes and hydrosiloxanes. ACS Catal 6:3589–3593
Bini L, Müller C, Vogt D (2010) Ligand development in the Ni-catalyzed hydrocyanation of alkenes. Chem Commun 46:8325–8334
Mifleur A, Mortreux A, Suisse I, Sauthier M (2017) Synthesis of C4 chain glyceryl ethers via nickel-catalyzed butadiene hydroalkoxylation reaction. J Mol Catal A: Chem 427:25–30
Mifleur A, Ledru H, Lopes A, Suisse I, Mortreux A, Sauthier M (2016) Synthesis of short-chain alkenyl ethers from primary and bio-sourced alcohols via the nickel-catalyzed hydroalkoxylation reaction of butadiene and derivatives. Adv Synth Catal 358:110–121
Bigot S, Ibn El Alami MS, Mifleur A, Castanet Y, Suisse I, Mortreux A, Sauthier M (2013) Nickel-catalysed hydroalkoxylation reaction of 1,3-butadiene: ligand controlled selectivity for the efficient and atom-economical synthesis of alkylbutenyl ethers. Chem Eur J 19:9785–9788
Nie SZ, Davison RT, Vy MD (2018) Enantioselective coupling of dienes and phosphine oxides. J Am Chem Soc 140:16450–16454
Mirzaei F, Han L, Tanaka M (2001) Palladium-catalyzed hydrophosphorylation of 1, 3-dienes leading to allylphosphonates. Tetrahedron Lett 42:297–299
Banerjee D, Junge K, Beller M (2014) Palladium-catalysed regioselective hydroamination of 1,3-dienes: synthesis of allylic amines. Org Chem Front 1:368–372
Goldfogel MJ, Roberts CC, Meek SJ (2014) Intermolecular hydroamination of 1,3-dienes catalyzed by bis(phosphine)carbodicarbene-rhodium complexes. J Am Chem Soc 136:6227–6230
Yang XH, Lu A, Dong VM (2017) Intermolecular hydroamination of 1,3-dienes to generate homoallylic amine. J Am Chem Soc 139:14049–14052
Yang XH, Dong VM (2017) Rhodium-catalyzed hydrofunctionalization: enantioselective coupling of indolines and 1, 3-dienes. J Am Chem Soc 139:1774–1777
Banerjee D, Junge K, Beller M (2014) A general catalytic hydroamidation of 1,3-dienes: atom-efficient synthesis of N-allyl heterocycles, amides and sulphonamides. Angew Chem Int Ed 53:1630–1635
Satoh M, Nomoto Y, Miyaura N, Suzuki A (1989) New convenient approach to the preparation of (Z)-allylic boronates via catalytic 1, 4-hydroboration of 1, 3-dienes with catecholborane. Tetrahedron Lett 30:3789–3792
Trost BM (1995) Atom economy-a challenge for organic synthesis: homogeneous catalysis leads the way. Angew Chem Int Ed 34:259–281
Fassbach TA, Vorholt AJ, Leitner W (2019) The telomerization of 1, 3-dienes-A reaction grows up. ChemCatChem 11:1153–1166
Dewhirst KC (1967) Reaction of rhodium trichloride with dienes. J Org Chem 32:1297–1300
Kawazura H, Ohmori T (1972) On the catalytic addition of alcohols to 1, 3-butadiene with rhodium trichloride hydrates. Bull Chem Soc Jpn 45:2213–2214
Kawazura H, Takagaki T, Ishii Y (1975) The regioselective addition of alcohols to isoprene with rhodium trichloride hydrates. Bull Chem Soc Jpn 48:1949–1950
Fache E, Mercier C (1993) Addition of alcohols to isoprene catalyzed by hydrated rhodium trichloride: a way to improve the 1, 4-addition regioselectivity. J Mol Catal 78:21–30
Patrini R, Lami M, Marchionna M, Benvenuti F, Galleti AMR, Sbrana G (1998) Selective synthesis of octadienyl and butenyl ethers via reaction of 1, 3-butadiene with alcohols catalyzed by homogeneous palladium complexes. J Mol Catal 129(2–3):179–189
Utsunomiya M, Kawatsura M, Hartwig JF (2003) Palladium-catalyzed equilibrium addition of acidic OH groups across dienes. Angew Chem Int Ed 42:5865–5868
Shields TC, Walker WE (1971) Nickel-complex catalyzed telomerisation of butadiene with alcohols. J Chem Soc D 193–194.
Weigert FJ, Drinkard WC (1973) The Ni(0)-catalyzed addition of phenol to butadiene. J Org Chem 38:335–337
Beger J, Duschek C, Füllbier H, Gaube W (1974) Diene oligomerization. IX. Nickel complex-catalyzed dimerization and telomerization of butadiene in hydroxy group-containing media. J Prakt Chem 316:26–42
Commereuc D, Chauvin Y (1974) Telomerization of butadiene with methanol using homogeneous catalysts of palladium and nickel. Bull Soc Chim Fr 3–4:652–656
Boelens H, Ter Heide R, Rijkens F (1968) Chemical structure and odor. VI Geraniol derivatives Am Perfum Cosmet 83:27–30
Behr A, Johnen L, Neubert P (2012) A sustainable route from the renewable myrcene to methyl ethers via direct hydroalkoxylation. Catal Sci Technol 2:88–92
Williams DBG, Sibiya MS, Van Heerden PS (2012) Efficient hydroalkoxylation of alkenes to generate octane-boosting ethers using recyclable metal triflates and highly active metal triflate/Brønsted acid-assisted catalysts. Fuel Process Technol 94:75–79
Chandrasekhar B, Ryu JS (2012) Gold-catalyzed intramolecular hydroalkoxylation/cyclization of conjugated dienyl alcohols. Tetrahedron 68:4805–4812
Mifleur A, Mérel D, Mortreux A, Suisse I, Capet F, Trivelli X, Sauthier M, MacGregor SA (2017) Deciphering the mechanism of the nickel-catalyzed hydroalkoxylation reaction: a combined experimental and computational Study. ACS Catal 7:6915–6923
Trost BM, Crawley ML (2003) Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem Rev 103:2921–2943
Tran G, Mazet C (2019) Ni-catalyzed regioselective hydroalkoxylation of branched 1,3-dienes. Org Lett 21:9124–9127
Kumobayashi H, Miura T, Sayo N, Saito T, Zhang X. (2001). Recent advances of BINAP chemistry in the industrial aspects. Synlett, 1055–1064.
Trost BM, Van Vranken DL (1992) Asymmetric ligands for transition metal catalyzed reactions: 2-diphenylphosphinobenzoyl derivatives of C2 symmetrical diols and diamines. Angew Chem Int Ed 31:228–230
Acknowledgements
We are grateful to the Region Nord-Pas de Calais for A.M.’s fellowship. We acknowledge the Ministry of Research and Technology, the Institut Universitaire de France, the ANR (project H2CAT: ANR-15-CE07-0018–01) and the CNRS for their financial support. We would like to express our gratitude to Dr Léo Violet and Dr Carole Mutschler for their precious advices.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Alan Welch on the occasion of his retirement from Heriot-Watt University.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mifleur, A., Suisse, I., Mortreux, A. et al. Enantioselective Nickel Catalyzed Butadiene Hydroalkoxylation with Ethanol: from Experimental Results to Kinetics Parameters. Catal Lett 151, 27–35 (2021). https://doi.org/10.1007/s10562-020-03267-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03267-z