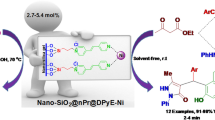
Abstract
An efficient supported ionic liquid catalyst is designed for condensation reaction of aldehydes and ketones. The Zr-based metal–organic framework (MOF), UiO-66-NH2, was initially functionalized with N,N′-dibutyl imidazolium ionic liquid (UiO-66-NH2-ILBr–), and then urea was attached to the ionic liquid (IL) to form a task-specific IL. Bromide was exchanged with tetrafluoroborate and the catalyst exhibits excellent performance for the synthesis of oximes. The ionic liquid/urea coupling showed a synergistic effect on the efficiency of the reaction. The supported catalyst system was recycled simply by filtration and reused for five times without significant decrease in its activity. The catalyst was characterized with PXRD, FTIR, TGA, XPS, BET, FE-SEM, EDS, elemental mapping and elemental analysis (CHN).
Graphic Abstract
MOF/IL/urea catalytic system was used for the synthesis of oximes








Similar content being viewed by others
References
Campisciano V, Giacalone F, Gruttadauria M (2017) Supported ionic liquids: a versatile and useful class of materials. Chem Rec 17:918–938
Romanovsky BV, Tarkhanova IG (2017) Supported ionic liquids in catalysis. Russ Chem Rev 86:444–458
Riisager A, Jørgensen B, Wasserscheid P, Fehrmann R (2006) First application of supported ionic liquid phase (SILP) catalysis for continuous methanol carbonylation. Chem Commun 9:994–996
Joni J, Haumann M, Wasserscheid P (2009) Development of a supported ionic liquid phase (SILP) catalyst for slurry-phase Friedel-Crafts alkylations of cumene. Adv Synth Catal 351:423–431
Lemus J, Palomar J, Gilarranz MA, Rodriguez JJ (2011) Characterization of supported ionic liquid phase (SILP) materials prepared from different supports. Adsorption 17:561–571
More S, Jadhav S, Salunkhe R, Kumbhar A (2017) Palladium supported ionic liquid phase catalyst (Pd@SILP-PS) for room temperature Suzuki-Miyaura cross-coupling reaction. Mol Catal 442:126–132
Xin B, Hao J (2014) Imidazolium-based ionic liquids grafted on solid surfaces. Chem Soc Rev 43:7171–7187
Riisager A, Fehrmann R, Haumann M, Wasserscheid P (2006) Supported ionic liquid phase (SILP) catalysis: An innovative concept for homogeneous catalysis in continuous fixed-bed reactors. Eur J Inorg Chem 2006:695–706
Zhang Q, Zhang S, Deng Y (2011) Recent advances in ionic liquid catalysis. Green Chem 13:2619–2637
Hagiwara H, Sekifuji M, Hoshi T, Qiao K, Yokoyama C (2007) Synthesis of bis(indolyl)methanes catalyzed by acidic ionic liquid immobilized on silica (ILIS). Synlett 2007:1320–1322
Gu Y, Li G (2009) Ionic liquids-based catalysis with solids: state of the art. Adv Synth Catal 351:817–847
Lee S-G (2006) Functionalized imidazolium salts for task-specific ionic liquids and their applications. Chem Commun 10:1049–1063
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl Catal A 373:1–56
Sawant AD, Raut DG, Darvatkar NB, Salunkhe MM (2011) Recent developments of task-specific ionic liquids in organic synthesis. Green Chem Lett Rev 4:41–54
Izák P, Bobbink FD, Hulla M, Klepic M, Friess K, Hovorka S, Dyson PJ (2018) Catalytic ionic-liquid membranes: the convergence of ionic-liquid catalysis and ionic-liquid membrane separation technologies. ChemPlusChem 83:7–18
Mehnert CP (2005) Supported ionic liquid catalysis. Chem Eur J 11:50–56
Mehnert CP, Cook RA, Dispenziere NC, Afeworki M (2002) Supported ionic liquid catalysis—a new concept for homogeneous hydroformylation catalysis. J Am Chem Soc 124:12932–12933
Li H, Bhadury PS, Song B, Yang S (2012) Immobilized functional ionic liquids: efficient, green, and reusable catalysts. RSC Adv 2:12525–12551
Luo Q-X, An B-W, Ji M, Zhang J (2018) Hybridization of metal–organic frameworks and task-specific ionic liquids: fundamentals and challenges. Mater Chem Front 2:219–234
Takashima Y, Yokoyama M, Horikoshi A, Sato Y, Tsuruoka T, Akamatsu K (2017) Ionic liquid/metal–organic framework hybrid generated by ion-exchange reaction: synthesis and unique catalytic activity. New J Chem 41:14409–14413
Farrusseng D, Aguado S, Pinel C (2009) Metal–organic frameworks: Opportunities for catalysis. Angew Chem Int Ed 48:7502–7513
He H, Perman JA, Zhu G, Ma S (2016) Metal-organic frameworks for CO2 chemical transformations. Small 12:6309–6324
Jiao L, Seow JYR, Skinner WS, Wang ZU, Jiang H-L (2019) Metal–organic frameworks: structures and functional applications. Mater Today 27:43–68
Luo Q-X, Ji M, Lu M-H, Hao C, Qiu J-S, Li Y-Q (2013) Organic electron-rich N-heterocyclic compound as a chemical bridge: building a Brönsted acidic ionic liquid confined in MIL-101 nanocages. J Mater Chem A 1:6530–6534
Luo Q-X, Ji M, Park S-E, Hao C, Li Y-Q (2016) PdCl2 immobilized on metal–organic framework CuBTC with the aid of ionic liquids: enhanced catalytic performance in selective oxidation of cyclohexene. RSC Adv 6:33048–33054
Abednatanzi S, Leus K, Gohari Derakhshandeh P, Nahra F, De Keukeleere K, Van Hecke K, Van Driessche I, Abbasi A, Nolan SP, Van Der Voort P (2017) POM@IL-MOFs—inclusion of POMs in ionic liquid modified MOFs to produce recyclable oxidation catalysts. Catal Sci Technol 7:1478–1487
Abednatanzi S, Abbasi A, Masteri-Farahani M (2017) Immobilization of catalytically active polyoxotungstate into ionic liquid-modified MIL-100(Fe): a recyclable catalyst for selective oxidation of benzyl alcohol. Catal Commun 96:6–10
Khan NA, Hasan Z, Jhung SH (2014) Ionic liquids supported on metal-organic frameworks: remarkable adsorbents for adsorptive desulfurization. Chem Eur J 20:376–380
Wu J, Gao Y, Zhang W, Tan Y, Tang A, Men Y, Tang B (2015) Deep desulfurization by oxidation using an active ionic liquid-supported Zr metal–organic framework as catalyst. Appl Organometal Chem 29:96–100
Luo Q-X, Song X-D, Ji M, Park S-E, Hao C, Li Y-Q (2014) Molecular size- and shape-selective Knoevenagel condensation over microporous Cu3(BTC)2 immobilized amino-functionalized basic ionic liquid catalyst. Appl Catal A 478:81–90
Tharun J, Bhin K-M, Roshan R, Kim DW, Kathalikkattil AC, Babu R, Ahn HY, Won YS, Park D-W (2016) Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2. Green Chem 18:2479–2487
Ding L-G, Yao B-J, Jiang W-L, Li J-T, Fu Q-J, Li Y-A, Liu Z-H, Ma J-P, Dong Y-B (2017) Bifunctional imidazolium-based ionic liquid decorated UiO-67 type MOF for selective CO2 adsorption and catalytic property for CO2 cycloaddition with epoxides. Inorg Chem 56:2337–2344
Ma D, Li B, Liu K, Zhang X, Zou W, Yang Y, Li G, Shi Z, Feng S (2015) Bifunctional MOF heterogeneous catalysts based on the synergy of dual functional sites for efficient conversion of CO2 under mild and co-catalyst free conditions. J Mater Chem A 3:23136–23142
Wu Z, Chen C, Wan H, Wang L, Li Z, Li B, Guo Q, Guan G (2016) Fabrication of magnetic NH2-MIL-88B (Fe) confined Brønsted ionic liquid as an efficient catalyst in biodiesel synthesis. Energy Fuels 30:10739–10746
Yang SH, Chang S (2001) Highly efficient and catalytic conversion of aldoximes to nitriles. Org Lett 3:4209–4211
Park S, Choi Y-A, Han H, Yang SH, Chang S (2003) Rh-Catalyzed one-pot and practical transformation of aldoximes to amides. Chem Commun 15:1936–1937
Peng H-G, Xu L, Wu H, Zhang K, Wu P (2013) One-pot synthesis of benzamide over a robust tandem catalyst based on center radially fibrous silica encapsulated TS-1. Chem Commun 49:2709–2711
Klitgaard SK, Egeblad K, Mentzel UV, Popov AG, Jensen T, Taarning E, Nielsen IS, Christensen CH (2008) Oxidations of amines with molecular oxygen using bifunctional gold–titania catalysts. Green Chem 10:419–423
Hajipour AR, Mallakpour SE, Imanzadeh G (1999) A rapid and convenient synthesis of oximes in dry media under microwave irradiation. J Chem Res (S) 3:228–229
Hajipour AR, Mallakpour SE, Khoee S (2002) An easy and fast method for conversion of oximes to the corresponding carbonyl compounds under microwave irradiation. Synth Commun 32:9–15
Kukushkin VY, Pombeiro AJL (1999) Oxime and oximate metal complexes: unconventional synthesis and reactivity. Coord Chem Rev 181:147–175
Li J-T, Li X-L, Li T-S (2006) Synthesis of oximes under ultrasound irradiation. Ultrason Sonochem 13:200–202
Sridhar M, Narsaiah C, Raveendra J, Reddy GK, Reddy MKK, Ramanaiah BC (2011) Efficient microwave-assisted synthesis of oximes from acetohydroxamic acid and carbonyl compounds using BF3·OEt2 as the catalyst. Tetrahedron Lett 52:4701–4704
Sha Q, Wei Y (2013) Base and solvent mediated decomposition of tosylhydrazones: highly selective synthesis of N-alkyl substituted hydrazones, dialkylidenehydrazines, and oximes. Tetrahedron 69:3829–3835
Yu J, Lu M (2015) Copper(II)-promoted direct conversion of methylarenes into aromatic oximes. Org Biomol Chem 13:7397–7401
Yu J, Jin Y, Lu M (2015) 3-Methyl-4-oxa-5-azahomoadamantane as an organocatalyst for the aerobic oxidation of primary amines to oximes in water. Adv Synth Catal 357:1175–1180
Xue X, Song F, Ma B, Yu Y, Li C, Ding Y (2013) Selective ammoximation of ketones and aldehydes catalyzed by a trivanadium-substituted polyoxometalate with H2O2 and ammonia. Catal Commun 33:61–65
Xing S, Han Q, Shi Z, Wang S, Yang PP, Wu Q, Li M (2017) A hydrophilic inorganic framework based on a sandwich polyoxometalate: unusual chemoselectivity for aldehydes/ketones with in situ generated hydroxylamine. Dalton Trans 46:11537–11541
Hyodo K, Togashi K, Oishi N, Hasegawa G, Uchida K (2016) Brønsted acid catalyzed transoximation reaction: synthesis of aldoximes and ketoximes without use of hydroxylamine salts. Green Chem 18:5788–5793
Guo J-J, Jin T-S, Zhang S-L, Li T-S (2001) TiO2/SO42−: an efficient and convenient catalyst for preparation of aromatic oximes. Green Chem 3:193–195
Sloboda-Rozner D, Neumann R (2006) Aqueous biphasic catalysis with polyoxometalates: oximation of ketones and aldehydes with aqueous ammonia and hydrogen peroxide. Green Chem 8:679–681
Kad GL, Bhandari M, Kaur J, Rathee R, Singh J (2001) Solventless preparation of oximes in the solid state and via microwave irradiation. Green Chem 3:275–277
Aakeröy CB, Sinha AS, Epa KN, Spartz CL, Desper J (2012) A versatile and green mechanochemical route for aldehyde–oxime conversions. Chem Commun 48:11289–11291
Ren RX, Ou W (2001) Preparation of cyclic ketoximes using aqueous hydroxylamine in ionic liquids. Tetrahedron Lett 42:8445–8446
Zang H, Wang M, Cheng B-W, Song J (2009) Ultrasound-promoted synthesis of oximes catalyzed by a basic ionic liquid [bmIm]OH. Ultrason Sonochem 16:301–303
Hajipour AR, Rafiee F, Ruoho AE (2010) A rapid and convenient method for the synthesis of aldoximes under microwave irradiation using in situ generated ionic liquids. J Iran Chem Soc 7:114–118
Taylor MS, Jacobsen EN (2006) Asymmetric catalysis by chiral hydrogen-bond donors. Angew Chem Int Ed 45:1520–1543
Maheswara M, Siddaiah V, Gopalaiah K, Rao VM, Rao CV (2006) A simple and effective glycine-catalysed procedure for the preparation of oximes. J Chem Res 2006:362–363
Vitz J, Mac DH, Legoupy S (2007) Ionic liquid supported tin reagents for Stille cross coupling reactions. Green Chem 9:431–433
Zhang Y, Zhen B, Li H, Feng Y (2018) Basic ionic liquid as catalyst and surfactant: green synthesis of quinazolinone in aqueous media. RSC Adv 8:36769–36774
Kandiah M, Nilsen MH, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Quadrelli EA, Bonino F, Lillerud KP (2010) Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem Mater 22:6632–6640
Katz MJ, Brown ZJ, Colón YJ, Siu PW, Scheidt KA, Snurr RQ, Hupp JT, Farha OK (2013) A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem Commun 49:9449–9451
Zhang L, Chen H, Zha Z, Wang Z (2012) Electrochemical tandem synthesis of oximes from alcohols using KNO3 as the nitrogen source, mediated by tin microspheres in aqueous medium. Chem Commun 28:6574–6576
Hinde CS, Webb WR, Chew BKJ, Tan HR, Zhang W-H, Hor TSA, Raja R (2016) Utilisation of gold nanoparticles on amine-functionalised UiO-66 (NH2-UiO-66) nanocrystals for selective tandem catalytic reactions. Chem Commun 52:6557–6560
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87:1051–1069
Sui Z-Y, Cui Y, Zhu J-H, Han B-H (2013) Preparation of three-dimensional graphene oxide−polyethylenimine porous materials as dye and gas adsorbents. ACS Appl Mater Interfaces 5:9172–9179
Beattie DA, Arcifa A, Delcheva I, Le Cerf BA, MacWilliams SV, Rossi A, Krasowska M (2018) Adsorption of ionic liquids onto silver studied by XPS. Colloids Surf A 544:78–85
Li X, Sui Z-Y, Sun Y-N, Xiao P-W, Wang X-Y, Han B-H (2018) Polyaniline-derived hierarchically porous nitrogen-doped carbons as gas adsorbents for carbon dioxide uptake. Microporous Mesoporous Mater 257:85–91
Xiao P-W, Zhao L, Sui Z-Y, Han B-H (2017) Synthesis of core−shell structured porous nitrogen-doped carbon@silica material via a sol−gel method. Langmuir 33:6038–6045
Gupta R, Yadav M, Gaur R, Arora G, Sharma RK (2017) A straightforward one-pot synthesis of bioactive N-aryl oxazolidin-2-ones via a highly efficient Fe3O4@SiO2-supported acetate-based butylimidazolium ionic liquid nanocatalyst under metal- and solvent-free conditions. Green Chem 19:3801–3812
Elmakssoudi A, Abdelouahdi K, Zahouily M, Clark J, Solhy A (2012) Efficient conversion of aldehydes and ketones into oximes using a nanostructured pyrophosphate catalyst in a solvent-free process. Catal Commun 29:53–57
Ribeiro TS, Prates A, Alves SR, Oliveira-Silva JJ, Riehl CAS, Figueroa-Villar JD (2012) The effect of neutral oximes on the reactivation of human acetylcholinesterase inhibited with paraoxon. J Braz Chem Soc 23:1216–1225
Van Doorslaer C, Wahlen J, Mertens P, Binnemans K, De Vos D (2010) Immobilization of molecular catalysts in supported ionic liquid phases. Dalton Trans 39:8377–8390
Zhu A, Jiang T, Han B, Zhang J, Xie Y, Ma X (2007) Supported choline chloride/urea as a heterogeneous catalyst for chemical fixation of carbon dioxide to cyclic carbonates. Green Chem 9:169–172
Corma A, García H, Llabrés i Xamena FX (2010) Engineering metal organic frameworks for heterogeneous catalysis. Chem Rev 110:4606–4655
Chen J, Shen K, Li Y (2017) Greening the processes of metal–organic framework synthesis and their use in sustainable catalysis. Chemsuschem 10:3165–3187
Khoramabadi-Zad A, Azadmanesh M, Rezaee A (2010) Simple, Efficient and green synthesis of oximes under ultrasound irradiation. S Afr J Chem 63:192–194
Acknowledgements
M. Jafarzadeh is thankful to Razi University for the partial financial supports, and Prof. Kim Daasbjerg and Monica Rohde Madsen (Aarhus University, Denmark) for XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Askari, S., Jafarzadeh, M., Christensen, D.B. et al. A Synergic Activity of Urea/Butyl Imidazolium Ionic Liquid Supported on UiO-66-NH2 Metal–Organic Framework for Synthesis of Oximes. Catal Lett 150, 3159–3173 (2020). https://doi.org/10.1007/s10562-020-03203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03203-1