Abstract
Molybdenum carbide (Mo2C) based electrocatalysts have been considered one of the promising candidates to replace Pt-based catalysts toward the hydrogen evolution reaction (HER). However, their practical application remains a great challenge. In this report, Mo2C nanoparticles are supported on a high-defective graphene nanospheres (GNs) synthesized by arc in liquid method. Attributing to the defect-rich features of GNs, the supported Mo2C nanoparticles with diameters of ~ 1.87 nm exhibit a homogeneous dispersion without obvious aggregation. Accordingly, the Mo2C/GNs catalyst exhibits an improved HER activity compared to MoO2/GNs with smaller Tafel slope of 58.6 mV dec−1 and lower overpotential of 196 mV to reach − 10 mA cm−2, as well as excellent cycling stability. These findings present the potential to enhance the catalytic performance of Mo2C species by adopting defect-rich carbon supports.
Graphic Abstract
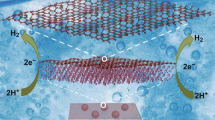
A high-defective GNs were successfully synthesized through a simple and rapid self-assembly strategy called “the arc-discharge in liquid toluene” method, and then used as the support for Mo2C without any pre-treatment. Attributing to the structural features, Mo2C nanoparticles are supported on the prepared GNs with diameters of ~ 1.87 nm exhibit a homogeneous dispersion without obvious aggregation. In addition, the Mo2C/GNs catalyst exhibits an improved HER activity compared to Mo2/GNs with smaller Tafel slope of 58.6 mV dec−1 and lower overpotential of 196 mV to reach −10 mA cm−2, as well as excellent cycling stability. These findings present the potential to enhance the catalytic performance of Mo2C species by defective carbon supports.






Similar content being viewed by others
References
Jo'M B (2002) The origin of ideas on a hydrogen economy and its solution to the decay of the environment. Int J Hydrogen Energy 27:731–740
Zou X, Zhang Y (2015) Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev 44:5148–5180
Nejat P, Jomehzadeh F, Taheri MM, Gohari M, Majid MZA (2015) A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew Sustain Energy Rev 43:843–862
McCrory CC, Jung S, Ferrer IM, Chatman SM, Peters JC, Jaramillo TF (2015) Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc 137:4347–4357
Cheng N, Stambula S, Wang D, Banis MN, Liu J, Riese A, Xiao B, Li R, Sham T-K, Liu L-M (2016) Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat Commun 7:13638
Yan X, Duan P, Zhang F, Li H, Zhang H, Zhao M, Zhang X, Xu B, Pennycook SJ, Guo J (2019) Stable single-atom platinum catalyst trapped in carbon onion graphitic shells for improved chemoselective hydrogenation of nitroarenes. Carbon 143:378–384
Li H, Zhang H-X, Yan X-L, Xu B-S, Guo J-J (2018) Carbon-supported metal single atom catalysts. New Carbon Mater 33:1–11
Yan X, Li H, Sun J, Liu P, Zhang H, Xu B, Guo J (2018) Pt nanoparticles decorated high-defective graphene nanospheres as highly efficient catalysts for the hydrogen evolution reaction. Carbon 137:405–410
Wu HB, Xia BY, Yu L, Yu X-Y, Lou XWD (2015) Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat Commun 6:6512
Zhu X, Liu M, Liu Y, Chen R, Nie Z, Li J, Yao S (2016) Carbon-coated hollow mesoporous FeP microcubes: an efficient and stable electrocatalyst for hydrogen evolution. J Mater Chem A 4:8974–8977
Laursen AB, Kegnæs S, Dahl S, Chorkendorff I (2012) Molybdenum sulfides—efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution. Energy Environ Sci 5:5577–5591
Park H, Encinas A, Scheifers JP, Zhang Y, Fokwa BP (2017) Boron-dependency of molybdenum boride electrocatalysts for the hydrogen evolution reaction. Angew Chem Int Ed 56:5575–5578
Xiao P, Sk MA, Thia L, Ge X, Lim RJ, Wang J-Y, Lim KH, Wang X (2014) Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ Sci 7:2624–2629
Zhang Y, Ouyang B, Xu J, Chen S, Rawat RS, Fan HJ (2016) 3D Porous hierarchical nickel-molybdenum nitrides synthesized by RF plasma as highly active and stable hydrogen-evolution-reaction electrocatalysts. Adv Energy Mater 6:1600221
Chen S, Duan J, Tang Y, Jin B, Qiao SZ (2015) Molybdenum sulfide clusters-nitrogen-doped graphene hybrid hydrogel film as an efficient three-dimensional hydrogen evolution electrocatalyst. Nano Energy 11:11–18
Mortensen PM, de Carvalho HW, Grunwaldt J-D, Jensen PA, Jensen AD (2015) Activity and stability of Mo2C/ZrO2 as catalyst for hydrodeoxygenation of mixtures of phenol and 1-octanol. J Catal 328:208–215
Guo J, Mao Z, Yan X, Su R, Guan P, Xu B, Zhang X, Qin G, Pennycook SJ (2016) Ultrasmall tungsten carbide catalysts stabilized in graphitic layers for high-performance oxygen reduction reaction. Nano Energy 28:261–268
Wan C, Regmi YN, Leonard BM (2014) Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew Chem Int Ed 53:6407–6410
Guo J, Wang J, Wu Z, Lei W, Zhu J, Xia K, Wang D (2017) Controllable synthesis of molybdenum-based electrocatalysts for a hydrogen evolution reaction. J Mater Chem A 5:4879–4885
Gao Q, Zhang W, Shi Z, Yang L, Tang Y (2019) Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution. Adv Mater 31:1802880
Li M, Zhou H, Yang W, Chen L, Huang Z, Zhang N, Fu C, Kuang Y (2017) Co9 S 8 nanoparticles embedded in a N S co-doped graphene-unzipped carbon nanotube composite as a high performance electrocatalyst for the hydrogen evolution reaction. J Mater Chem A 5:1014–1021
Zhu YP, Liu Y, Liu YP, Ren TZ, Chen T, Yuan ZY (2015) Direct synthesis of phosphorus-doped mesoporous carbon materials for efficient electrocatalytic oxygen reduction. ChemCatChem 7:2903–2909
Wu Z, Wang J, Liu R, Xia K, Xuan C, Guo J, Lei W, Wang D (2017) Facile preparation of carbon sphere supported molybdenum compounds (P, C and S) as hydrogen evolution electrocatalysts in acid and alkaline electrolytes. Nano Energy 32:511–519
Wei H, Xi Q, Chen XA, Guo D, Ding F, Yang Z, Wang S, Li J, Huang S (2018) Molybdenum carbide nanoparticles coated into the graphene wrapping n‐doped porous carbon microspheres for highly efficient electrocatalytic hydrogen evolution both in acidic and alkaline media. Adv Sci 5:1700733
Lv C, Huang Z, Yang Q, Zhang C (2018) Nanocomposite of MoO 2 and MoC loaded on porous carbon as an efficient electrocatalyst for hydrogen evolution reaction. Inorg Chem Front 5:446–453
Cheng Z, Gao J, Fu Q, Li C, Wang X, Xiao Y, Zhao Y, Zhang Z, Qu L (2017) Interconnected molybdenum carbide-based nanoribbons for highly efficient and ultrastable hydrogen evolution. ACS Appl Mater Interfaces 9:24608–24615
Xu B, Guo J, Wang X, Liu X, Ichinose H (2006) Synthesis of carbon nanocapsules containing Fe, Ni or Co by arc discharge in aqueous solution. Carbon 44:2631–2634
Li M, Guo J, Xu B (2013) Superelastic carbon spheres under high pressure. Appl Phys Lett 102:121904
Guo J, Wang X, Xu B (2009) One-step synthesis of carbon-onion-supported platinum nanoparticles by arc discharge in an aqueous solution. Mater Chem Phys 113:179–182
Guo J, Liu G, Wang X, Fujita T, Xu B, Chen M (2009) High-pressure Raman spectroscopy of carbon onions and nanocapsules. Appl Phys Lett 95:051920
Guo J, Lee J, Contescu CI, Gallego NC, Pantelides ST, Pennycook SJ, Moyer BA, Chisholm MF (2014) Crown ethers in graphene. Na Commun 5:5389
Guo J, Morris JR, Ihm Y, Contescu CI, Gallego NC, Duscher G, Pennycook SJ, Chisholm MF (2012) Topological defects: origin of nanopores and enhanced adsorption performance in nanoporous carbon. Small 8:3283–3288
Guo JJ, Morris JR, Ihm Y, Contescu CI, Gallego NC, Duscher G (2012) To-pological defects: origin of nanopores and enhanced adsorption performance in nanoporous carbon. Small 8:3283–3288
Youn DH, Han S, Kim JY, Kim JY, Park H, Choi SH, Lee JS (2014) Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube–graphene hybrid support. ACS Nano 8:5164–5173
Peng L, Zhang L, Shen J, Wei Z (2017) Self-assembly-and preshaping-assisted synthesis of ultrathin nitrogen-doped graphitic carbon lamellas supported molybdenum carbide for hydrogen evolution reaction, Meeting Abstracts, The Electrochemical Society, pp 2219–2219
Shi Z, Wang Y, Lin H, Zhang H, Shen M, Xie S, Zhang Y, Gao Q, Tang Y (2016) Porous nanoMoC@ graphite shell derived from a MOFs-directed strategy: an efficient electrocatalyst for the hydrogen evolution reaction. J Mater Chem A 4:6006–6013
Dong J, Wu Q, Huang C, Yao W, Xu Q (2018) Cost effective Mo rich Mo2C electrocatalysts for the hydrogen evolution reaction. J Mater Chem A 6:10028–10035
Vrubel H, Hu X (2012) Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew Chem Int Ed 51:12703–12706
Pan LF, Li YH, Yang S, Liu PF, Yu MQ, Yang HG (2014) Molybdenum carbide stabilized on graphene with high electrocatalytic activity for hydrogen evolution reaction. Chem Commun 50:13135–13137
Kunhiraman AK, Ramasamy M, Ramanathan S (2017) Efficient hydrogen evolution catalysis triggered by electrochemically anchored platinum nano-islands on functionalized-MWCNT. Int J Hydrogen Energy 42:9881–9891
Wu A, Tian C, Yan H, Jiao Y, Yan Q, Yang G, Fu H (2016) Hierarchical MoS 2@ MoP core–shell heterojunction electrocatalysts for efficient hydrogen evolution reaction over a broad pH range. Nanoscale 8:11052–11059
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1810204, 51602212, 51703150, 51701137), Basic Research Project in Shanxi Province (201901D211086, 201701D121043), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (STIP, 2019L0310, 2019L0120, 2019L0253, 2019L0346), and Program for the Innovative Talents of Higher Education Institutions in Shanxi, Special Foundation for Youth SanJin Scholars.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, X., Wang, D., Zhang, K. et al. Mo2C Decorated High-Defective Graphene Nanospheres for Improved Hydrogen Evolution Reaction Catalytic Performance. Catal Lett 150, 2141–2149 (2020). https://doi.org/10.1007/s10562-020-03134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03134-x