Abstract
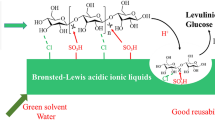
In this study, Zr-MOF material (UiO-66) was modified with different metal oxides (CeO2, Ga2O3, and CoO) and employed in the conversion of cellulose to levulinic acid (LA) in aqueous medium. The research results showed that the introduction of the metal oxides increased the acid content of the catalysts, especially in medium-strong acid. The selectivity of LA was related to the type of metal oxide and the content of medium-strong acid. Due to the activity of Ga2O3 and more medium-strong acid, the yield of LA reaches a maximum yield of 32.0 mol% at 513 K for 360 min when Ga2O3-UiO-66 was employed as the catalysts. The combination of metal oxide and UiO-66 enables the reaction to proceed, providing a new direction for the conversion of cellulose to LA in the aqueous phase.
Graphic Abstract
In this study, Zr-MOFs material (UiO-66) was modified with different metal oxides (CeO2, Ga2O3, and CoO) and employed in the conversion of cellulose to levulinic acid in aqueous medium. The results indicated that the addition of metal oxides changes the acidity of UiO-66, which improves the catalytic activity. The selectivity of LA was related to the type of metal oxide and the content of medium-strong acid.







Similar content being viewed by others
References
Lund H, Afgan H, Bogdan Z, Duić N, Guzović Z (2007) Energy 32:912–919
Balat M, Balat H (2010) Appl Energy 87:1815–1835
Vispute TP, Zhang H, Sanna A, Xiao R, Huber H (2010) Science 330:1222–1227
Kobayashi H, Ohta H, Fukuoka A (2012) Catal Sci Technol 2:869–883
Zhang Z, Huber GW (2018) Chem Soc Rev 47:1351–1390
Lin H, Strull J, Liu Y, Karmiol Z, Plank K, Miller G, Guo Z, Yang L (2012) Energy Environ Sci 5:9773
Bozell JJ, Moens L, Elliott DC, Wang Y, Neuenscwander GG, Fitzpatrick SW, Bilski RJ, Jarnefeld JL (2000) Resour Conserv Recycl 28:227–239
Fernandes DR, Rocha AS, Mai EF, Mota CJA, Silva VTD (2012) Appl Catal A 425–426:199–204
Serrano-Ruiz JC, Pineda A, Balu AM, Luque R, Campelo JM, Romero AA, Ramos-Fernández JM (2012) Catal Today 195:162–168
Matsumura Y, Sasaki M, Okuda K, Takami S, Ohara S, Umetsu M, Adschiri T (2006) Combust Sci Technol 178:509–536
Li Y-X, Wei Z-Y, Liu L, Gao M-L, Han Z-B (2018) Inorg Chem Commun 88:47–50
Weingarten R, Conner WC, Huber GW (2012) Energy Environ Sci 5:7559
Shen J, Wyman CE (2012) AIChE J 58:236–246
Peng L, Lin L, Zhang J, Zhuang J, Zhang B, Gong Y (2010) Molecules 15:5258–5272
Chiappe C, Douton MJR, Mezzetta A, Guazzelli L, Pomelli CS, Angelis AD, Assanelli G (2017) New J Chem 42(3):1845–1852
Wang P, Zhan SH, Yu HB (2010) Adv Mater Res 96:183–187
Xiang M, Liu J, Fu W, Tang T, Wu D (2017) ACS Sustain Chem Eng 5:5800–5809
Akiyama G, Matsuda R, Sato H, Takata M, Kitagawa S (2011) Adv Mater 23:3294–3297
Herbst A, Janiak C (2016) New J Chem 40:7958–7967
Su Y, Chang G, Zhang Z, Xing H, Su B, Yang Q, Ren Q, Yang Y, Bao Z (2016) AIChE J 62:4403–4417
Gong J, Katz MJ, Kerton FM (2018) RSC Adv 8:31618–31627
Zhang H, Nai J, Yu L, Lou XW (2017) Joule 1:77–107
Herbst A, Janiak C (2017) CrystEngComm 19:4092–4117
Katz MJ, Brown ZJ, Colón YJ, Siu PW, Scheidt KA, Snurr RQ, Hupp JT, Farha OK (2013) Chem Commun 49:9449–9451
Kandiah M, Nilsen MH, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Quadrelli EA, Bonino F, Lillerud KP (2010) Chem Mater 22:6632–6640
Sadeghi S, Jafarzadeh M, Reza Abbasi A, Daasbjerg K (2017) New J Chem 41:12014–12027
Insyani R, Verma D, Kim SM, Kim J (2017) Green Chem 19:2482–2490
Dong W, Feng C, Zhang L, Shang N, Gao S, Wang C, Wang Z (2015) Catal Lett 146:117–125
Lykaki M, Pachatouridou E, Carabineiro SAC, Iliopoulou E, Andriopoulou C, Kallithrakas-Kontos N, Boghosian S, Konsolakis M (2018) Appl Catal B 230:18–28
Zheng J, Lei Z (2018) Appl Catal B 237:1–8
Zeng W, Cheng DG, Zhang H, Chen F, Zhan X (2010) React Kinet Mech Catal 100:377–384
Jin F, Zhou Z, Enomoto H, Moriya T, Higashijima H (2004) Chem Lett 33:126–127
Wattanapaphawong P, Reubroycharoen P, Yamaguchi A (2017) RSC Adv 7:18561–18568
Acknowledgements
This study was supported financially by National Natural Science Foundation of China (No. 51708252), “the Fundamental Research Funds for the Central Universities” (No. 17lgpy71), National Science for Distinguished Young Scholars of China (No. 21425627), Guangdong Technology Research Center for Synthesis and Separation of Thermosensitive Chemicals (2015B090903061), and Science and Technology Innovation Teams Project of Huizhou (20131226121851953).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, K., Liu, Y., Wu, W. et al. Production of Levulinic Acid via Cellulose Conversion Over Metal Oxide-Loaded MOF Catalysts in Aqueous Medium. Catal Lett 150, 322–331 (2020). https://doi.org/10.1007/s10562-019-03023-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-03023-y