Abstract
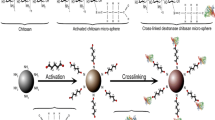
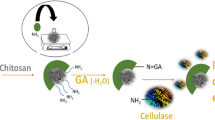
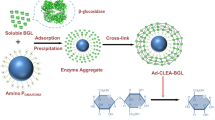
In the current research, second most abundant renewable polysaccharide chitin is utilized for meaningful purpose. Owing to auspicious features of chitin it is used as a matrix for immobilization of valuable biocatalyst dextranase. Dextranase belongs to hydrolase family and has broad commercial application in different fields. Functionality of dextranase is further improve with the help of its immobilization on chitin by different protocols. Dextranase used in this study was isolated from a thermophilic bacteria Bacillus megaterium KIBGE-IB31 explored from hydrothermal spring. Isolated dextranase was immobilized on chitin by two different methods namely; adsorption and covalent binding. A comparative study was conducted between soluble, adsorbed and covalently cross linked dextranase. It was observed that although, there was slight deviation in characteristics of dextranase after immobilization but there was marked improvement in stability of enzyme after immobilization. The comparative analysis revealed that dextranase immobilized by adsorption was less stable and fails to retained enzyme stability for increase reusability. The adsorption method was improved after treatment of chitin with acid and showed improved stability as well as reusability. However, dextranase immobilized by covalent cross linking displayed highest stability at high temperature along with increase recycling efficiency as compare to enzyme immobilized by adsorption method. Hence, it can be concluded that though chitin is cheap, easily accessible matrix for immobilization of dextranase but still among different protocols of immobilization covalent cross linking was found to be more appropriate in improving enzyme stability and its reusability at industrial scale.
Graphic Abstract





Similar content being viewed by others
References
Morganti P, Morganti G, Morganti A (2011) Transforming nanostructured chitin from crustacean waste into beneficial health products: a must for our society. Nanotechnol Sci Appl 4:123
Carroad PA, Tom RA (1978) Bioconversion of shellfish chitin wastes: process conception and selection of microorganisms. J Food Sci 43:1158–1161
Mao X, Guo N, Sun J, Xue C (2017) Comprehensive utilization of shrimp waste based on biotechnological methods: a review. J Clean Prod 143:814–823
Zdarta J, Klapiszewski Ł, Wysokowski M, Norman M, Kołodziejczak-Radzimska A, Moszyński D, Jesionowski T (2015) Chitin-lignin material as a novel matrix for enzyme immobilization. Mar Drugs 13:2424–2446
Nadezhda A, Krayukhina MA (2019) Synthesis of magnetic chitin–adsorbent for specific proteins. Carbohyd Polym 216:107–112
Batra R, Gupta MN (1994) Non-covalent immobilization of potato (Solanum tuberosum) polyphenol oxidase on chitin. Biotechnol Appl Biochem 21(2):223–230
Wang G, Xu JJ, Ye LH, Zhu JJ, Chen HY (2002) Highly sensitive sensors based on the immobilization of tyrosinase in chitosan. Bioelectrochemistry 57:33–38
Dutta PK, Dutta J, Tripathi VS (2004) Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res 63:20–31
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng Life Sci 13:49–61
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: review. Adsorption 20:801–821
Singh RS, Chauhan K (2019) Immobilization of inulinase on aminated multiwalled carbon nanotubes by glutaraldehyde cross-linking for the production of fructose. Catal Lett. https://doi.org/10.1007/s10562-019-02743-5
Szymańska K, Bryjak J, Jarzębski AB (2009) Immobilization of invertase on mesoporous silicas to obtain hyper active biocatalysts. Top Catal 52:1030–1036
Ispas C, Sokolov I, Andreescu S (2009) Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal Bioanal Chem 393:543–554
Khalikova E, Susi P, Korpela T (2005) Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol Mol Biol Rev 69:306–325
Jiménez ER (2009) Dextranase in sugar industry: a review. Int J Sugar Crops Related Ind 11:124–134
Otsuka R, Imai S, Murata T, Nomura Y, Okamoto M, Tsumori H, Momoi Y (2015) Application of chimeric glucanase comprising mutanase and dextranase for prevention of dental biofilm formation. Microbiol Immunol 59:28–36
Patel S, Goyal A (2011) World J Microbiol Biotechnol 27:1119–1128
Shahid F, Aman A, Pervez S, Qader SAU (2019) Degradation of long chain polymer (Dextran) using thermostable dextranase from hydrothermal spring isolate (Bacillus megaterium). Geomicrobiol J. https://doi.org/10.1080/01490451.2019.1605427
Shahid F, Aman A, Nawaz MA, Karim A, Qader SAU (2017) Chitosan hydrogel microspheres: an effective covalent matrix for crosslinking of soluble dextranase to increase stability and recycling efficiency. Bioprocess Biosyst Eng 40:451–461
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153(375–380):23
Somogyi M (1937) A reagent for the copper-iodometric determination of very small amounts of sugar. J Biol Chem 117:771–776
Lowry OH, Rosembrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Spain SG, Cameron NR (2011) A spoonful of sugar: the application of glycopolymers in therapeutics. Polym Chem 2:60–68
Marcin HS (2002) Chitin and chitosan. Polimery 47:396–402
Wang Y, Li Y, Liu S, Li B (2015) Fabrication of chitin microspheres and their multipurpose application as catalyst support and adsorbent. Carbohyd Polym 120:53–59
Nguyen VD, Styevkó G, Madaras E, Haktanirlar G, Tran AT, Bujna E, Nguyen QD (2019) Immobilization of β-galactosidase on chitosan-coated magnetic nanoparticles and its application for synthesis of lactulose-based galactooligosaccharides. Process Biochem 84:30–38
Movahedi M, Shariat SZAS, Nazem H (2019) Immobilization of lactoperoxidase on graphene oxide nanosheets and copper oxide nanoparticles and evaluation of their stability. Catal Lett 149(2):562–573
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235
Kennedy JF, Cabral JMS (1983) Immobilized enzymes. In: Scouten WH (ed) Solid Phase Biochemistry: Analytical and Synthetic Aspects. Wiley, New York, pp 253–391
Torchilin VP, Maksimenko AV, Smirknov VN, Berezin IV, Klibanov AM, Martinek K (1978) The principles of enzyme stabilization. III. The effect of the length of intra-molecular cross-linkages on thermostability of enzymes. Biochim et Biophys Acta 522:277–283
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–806
Acknowledgements
This research was funded and supported by The Karachi Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahid, F., Ansari, A., Aman, A. et al. A Comparative Study Among Different Protocols of Immobilization of Dextranase Using Chitin as a Matrix. Catal Lett 150, 613–622 (2020). https://doi.org/10.1007/s10562-019-02940-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02940-2