Abstract
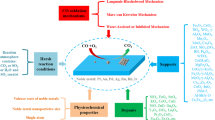
Ethanol conversion into n-butanol was performed over ZrO2–CeO2 mixed oxide catalysts. The effect of ceria content on the acid–base and catalytic properties of ZrO2–CeO2 compositions has been studied. The introduction of CeO2 additives into zirconia stabilizes the tetragonal phase of ZrO2 leading to an increase in basicity of the samples and, as a result, increases the activity of catalysts towards n-butanol. The highest ethanol conversion, selectivity and the rate of n-butanol formation were obtained over the ZrO2–CeO2 sample with 10% of ceria, which exhibited the highest concentration and strength of base sites. This sample consists of a solid solution of ZrO2–CeO2, whereas at higher concentrations of additive, CeO2 forms an individual phase.
Graphic Abstract






Similar content being viewed by others
References
Posada JA, Patel AD, Roes A et al (2013) Potential of bioethanol as a chemical building block for biorefineries: preliminary sustainability assessment of 12 bioethanol-based products. Bioresour Technol 135:490–499. https://doi.org/10.1016/j.biortech.2012.09.058
Mandegari M, Farzad S, Görgens J (2017) Recent trends on techno-economic assessment (TEA) of sugarcane biorefineries. Biofuel Res J 4(3):704–712. https://doi.org/10.18331/BRJ2017.4.3.7
Sun J, Wang Y (2014) Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal 4(4):1078–1090. https://doi.org/10.1021/cs4011343
Sun Z, Vasconcelos AC, Bottari G et al (2017) Efficient catalytic conversion of ethanol to 1-butanol via the guerbet reaction over copper- and nickel-doped porous. ACS Sustain Chem Eng 5(2):1738–1746. https://doi.org/10.1021/acssuschemeng.6b02494
Kozlowski JT, Davis RJ (2013) Heterogeneous catalysts for the Guerbet coupling of alcohols. ACS Catal 3(7):1588–1600. https://doi.org/10.1021/cs400292f
Kolesinska B, Fraczyk J, Binczarski M et al (2019) Butanol synthesis routes for biofuel production: trends and perspectives. Materials 12:350. https://doi.org/10.3390/ma12030350
Uyttebroek M, van Hecke W, Vanbroekhoven K (2015) Sustainability metrics of 1-butanol. Catal Today 239(1):7–10. https://doi.org/10.1016/j.cattod.2013.10.094
Wu X, Fang G, Tong Y et al (2018) Catalytic upgrading of ethanol to n-butanol: progress in catalyst development. Chem Sus Chem 11(1):71–85. https://doi.org/10.1002/cssc.201701590
Gabriëls D, Hernández WY, Sels B et al (2015) Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal Sci Technol 5(8):3876–3902. https://doi.org/10.1039/C5CY00359H
Galadima A, Muraza O (2015) Catalytic upgrading of bioethanol to fuel grade biobutanol: a review. Ind Eng Chem Res 54(29):7181–7194. https://doi.org/10.1021/acs.iecr.5b01443
Cimino S, Lisi L, Romanucci S (2018) Catalysts for conversion of ethanol to butanol: effect of acid-base and redox properties. Catal Today 304:58–63. https://doi.org/10.1016/j.cattod.2017.08.035
Kozlowski JT, Davis RJ (2013) Sodium modification of zirconia catalysts for ethanol coupling to 1-butanol. J Energy Chem 22(1):58–64. https://doi.org/10.1016/S2095-4956(13)60007-8
Kozlowski JT, Behrens M, Schlögl R, Davis RJ (2013) Influence of the precipitation method on acid–base-catalyzed reactions over Mg–Zr mixed oxides. Chem Cat Chem 5(7):1989–1997. https://doi.org/10.1002/cctc.201200833
Tanabe K, Yamaguchi T (1994) Acid-base bifunctional catalysis by ZrO2 and its mixed oxides. Catal Today 20(2):185–198. https://doi.org/10.1016/0920-5861(94)80002-2
Vlasenko NV, Kyriienko PI, Valihura KV et al (2019) Effect of modifying additives on the catalytic properties of zirconium dioxide in the conversion of ethanol into 1-butanol. Theor Exp Chem 55(1):43–49. https://doi.org/10.1007/s11237-019-09594-6
Shutilov A, Simonov MN, Zaytseva YA et al (2013) Phase composition and catalytic properties of ZrO2 and CeO2-ZrO2 in the ketonization of pentanoic acid to 5-nonanone. Kinet Catal 54(2):184–192. https://doi.org/10.1134/S0023158413020134
Kochkin YN, Vlasenko NV, Struzhko VL et al (2016) Methanol carboxylation over zirconium dioxide: effect of catalyst phase composition on its acid-base spectrum and direction of catalytic transformations. Can J Chem Eng 94(4):745–751. https://doi.org/10.1002/cjce.22435
Albuquerque EM, Borges LEP, Fraga MA, Sievers C (2017) Relationship between acid-base properties and the activity of ZrO2-based catalysts for the Cannizzaro reaction of pyruvaldehyde to lactic acid. Chem Cat Chem 9(14):2675–2683. https://doi.org/10.1002/cctc.201700305
Di Monte R, Kaspar J (2005) Nanostructured CeO2–ZrO2 mixed oxides. J Mater Chem 15(6):633–648. https://doi.org/10.1039/B414244F
Kašpar J, Fornasiero P (2003) Nanostructured materials for advanced automotive de-pollution catalysts. J Solid State Chem 171(1–2):19–29. https://doi.org/10.1016/S0022-4596(02)00141-X
Zavodinsky VG, Chibisov AN (2006) Stability of cubic zirconia and of stoichiometric zirconia nanoparticles. Phys Solid State 48(2):343–368. https://doi.org/10.1134/S1063783406020296
Ma Z-Y, Yang C, Wei W et al (2005) Surface properties and CO adsorption on zirconia polymorphs. J Mol Catal A 227(1–2):119–124. https://doi.org/10.1016/j.molcata.2004.10.017
Madier Y, Descorme C, Le Govic AM et al (1999) Oxygen mobility in CeO2 and CexZr(1–x)O2 compounds: study by CO transient oxidation and 18O/16O isotopic exchange. J Phys Chem B 103(50):10999–11006. https://doi.org/10.1021/jp991270a
Sergent N, Lamonier JF, Aboukais A (2000) Electron paramagnetic resonance in combination with the thermal analysis, X-ray diffraction, and raman spectroscopy to follow the structural properties of ZrxCe1-xO2 solid systems and precursors. Chem Mater 12(12):3830–3835. https://doi.org/10.1021/cm000315d
Montini T, Melchionna M, Monai M, Fornasiero P (2016) Fundamentals and catalytic applications of CeO2-based materials. Chem Rev 116(10):5987–6041. https://doi.org/10.1021/acs.chemrev.5b00603
Zhang X, Cheng X, Ma Ch et al (2018) Effect of ZrO2 support on Cu/Fe2O3-CeO2/ZrO2 catalyst for NO removal by CO using a rotary reactor. Catal Sci Technol 8(21):5623–5631. https://doi.org/10.1039/C8CY01546E
Fornasiero P, Ranga Rao G, Kašpar J et al (1998) Reduction of NO by CO over Rh/CeO2–ZrO2 catalysts: evidence for a support-promoted catalytic activity. J Catal 175(2):269–279. https://doi.org/10.1006/jcat.1998.1999
Ning P, Song Zh, Li H et al (2015) Selective catalytic reduction of NO with NH3 over CeO2–ZrO2–WO3 catalysts prepared by different methods. Appl Surf Sci 332:130–137. https://doi.org/10.1016/j.apsusc.2015.01.118
Putluru SSR, Riisager A, Fehrmann R (2009) The effect of acidic and redox properties of V2O5/CeO2–ZrO2 catalysts in selective catalytic reduction of NO by NH3. Catal Lett 133(3–4):370–375. https://doi.org/10.1007/s10562-009-0176-8
Haneda M, Taguchi R, Hattori M (2015) Influence of particle morphology on catalytic performance of CeO2/ZrO2 for soot oxidation. J Ceram Soc Jpn 123(1437):414–418. https://doi.org/10.2109/jcersj2.123.414
Atzori L, Rombi E, Meloni D et al (2019) CO and CO2 Co-methanation on Ni/CeO2-ZrO2 soft-templated catalysts. Catalysts 9(5):415. https://doi.org/10.3390/catal9050415
Kambolis A, Matralis H, Trovarelli A, Papadopoulou Ch (2010) Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl Catal A 377(1–2):16–26. https://doi.org/10.1016/j.apcata.2010.01.013
Wolfbeisser A, Sophiphun O, Bernardi J et al (2016) Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal Today 277(2):234–245. https://doi.org/10.1016/j.cattod.2016.04.025
Palikanon T, Laosiripojana N, Assabumrungrat S, Charojrochkul S (2006) Hydrogen production from methane steam reforming over Ni on high surface area CeO2 and CeO2-ZrO2 supports synthesized by surfactant-assisted method. Songklanakarin J Sci Technol 28(6): 1238–1249. http://rdo.psu.ac.th/sjstweb/journal/28-6/10-Methane.pdf
Palma V, Ruocco C, Meloni E, Ricca A (2017) Renewable hydrogen from ethanol reforming over CeO2-SiO2 based catalysts. Catalysts 7:226. https://doi.org/10.3390/catal7080226
Palma V, Ruocco C, Ricca A (2016) Ceramic foams coated with PteNi/CeO2-ZrO2 for bioethanol steam reforming. Int J Hydrog Energy 41(27):11526–11536. https://doi.org/10.1016/j.ijhydene.2016.04.028
Palma V, Ruocco C, Ricca A (2016) Low-temperature steam reforming of raw bio-ethanol over ceria-zirconia supported catalysts. Chem Eng Trans 52:193–198. https://doi.org/10.3303/CET1652033
Perdomo Rodrigues C, da Costa Zonetti P, Gorenstin Appel L (2017) Chemicals from ethanol: the acetone synthesis from ethanol employing Ce0.75Zr0.25O2, ZrO2 and Cu/ZnO/Al2O3. Chem Cent J 11: 30. https://doi.org/10.1186/s13065-017-0249-5
Birot A, Epron F, Descorme C, Duprez D (2008) Ethanol steam reforming over Rh/CexZr1−xO2 catalysts: impact of the CO–CO2–CH4 interconversion reactions on the H2 production. Appl Catal B 79(1):17–25. https://doi.org/10.1016/j.apcatb.2007.10.002
Biswas P, Kunzru D (2007) Steam reforming of ethanol for production of hydrogen over Ni/CeO2–ZrO2 catalyst: effect of support and metal loading. Int J Hydrog Energy 32(8):969–980. https://doi.org/10.1016/j.ijhydene.2006.09.031
Waseda Y, Matsubara E, Shinoda K (2011) X-ray diffraction crystallography. Springer, Berlin. ISBN 978-3-642-16634-1
Toraya H, Yoshimura M, Somiya S (1984) Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction. J Am Ceram Soc 67(6):119–121. https://doi.org/10.1111/j.1151-2916.1984.tb19715.x
Masudi A, Muraza O (2018) Zirconia-based nanocatalysts in heavy oil upgrading: a mini review. Energy Fuel 32(2):2840–2854. https://doi.org/10.1021/acs.energyfuels.7b03264
Viinikainen T, Ronkkonen H, Bradshaw H et al (2009) Acidic and basic surface sites of zirconia-based biomass gasification gas clean-up catalysts. Appl Catal A 362(1–2):169–177. https://doi.org/10.1016/j.apcata.2009.04.037
Bachiller-Baeza B, Rodriguez-Ramos I, Guerrero-Ruiz A (1998) Interaction of carbon dioxide with the surface of zirconia polymorphs. Langmuir 14(13):3556–3564. https://doi.org/10.1021/la970856q
Panchenko VN, Paukshtis EA, Murzin DY, Simakova IL (2017) Solid base assisted n-pentanol coupling over VIII group metals: elucidation of the guerbet reaction mechanism by DRIFTS. Ind Eng Chem Res 56(45):13310–13321. https://doi.org/10.1021/acs.iecr.7b01853
Ho CR, Shylesh S, Bell AT (2016) Mechanism and kinetics of ethanol coupling to butanol over hydroxyapatite. ACS Catal 6(2):939–948. https://doi.org/10.1021/acscatal.5b02672
Wang R, Lan L, Gong M-C, Chen Y-Q (2016) Catalytic combustion of gasoline particulate soot over CeO2-ZrO2 catalysts. Acta Phys-Chim Sin 32(7):1747–1757. https://doi.org/10.3866/pku.whxb201605103
Olcese R, Bettahar MM (2013) Thermodynamics conditions for Guerbet ethanol reaction. MATEC Web Conf 3:01060. https://doi.org/10.1051/matecconf/20130301060
Riittonen T, Toukoniitty E, Madnani DK et al (2012) One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide—the effect of the active metal on the selectivity. Catalysts 2:68–84. https://doi.org/10.3390/catal2010068
Jordison TL, Peereboom L, Miller DJ (2016) Impact of water on condensed phase ethanol guerbet reactions. Ind Eng Chem Res 55(23):6579–6585. https://doi.org/10.1021/acs.iecr.6b00700
Zaytseva YA, Panchenko VN, Simonov MN et al (2013) Effect of gas atmosphere on catalytic behaviour of zirconia, ceria and ceria–zirconia catalysts in valeric acid ketonization. Top Catal 56(9–10):846–855. https://doi.org/10.1007/s11244-013-0045-y
Acknowledgements
The authors are grateful to Dr. V.L. Struzhko for the synthesis of ZrO2–CeO2 samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vlasenko, N.V., Kyriienko, P.I., Yanushevska, O.I. et al. The Effect of Ceria Content on the Acid–Base and Catalytic Characteristics of ZrO2–CeO2 Oxide Compositions in the Process of Ethanol to n-Butanol Condensation. Catal Lett 150, 234–242 (2020). https://doi.org/10.1007/s10562-019-02937-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02937-x