Abstract
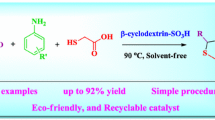
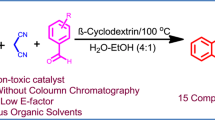
Green and economical method has been reported for the synthesis of benzylpyrazolyl naphthoquinone and pyrazolo pyranopyrimidines in water at room temperature by using β-CD-SO3H. β-Cyclodextrin supported sulfonic acid was prepared by simple one step procedure and characterized by FT-IR spectrum, 1H NMR, 13C NMR spectra, TGA, EDAX, XRD, BET surface area analysis and acid–base titration. The present protocol is environmental benign due to heterogeneous reusable catalyst and green reaction medium. This methodology provides excellent yield of the desired product with short reaction time at room temperature, easy workup procedure and no need of column chromatographic separation. Pyrazolyl derivatives are of much importance because this fragment is a key moiety in numerous biologically active compounds.
Graphic Abstract













Similar content being viewed by others
References
Ruhul AM, Kalam MA, Masjuki HH, Fattah IMR, Reham SS, Rashed MM (2015) RSC Adv 5:101023–101044
Zhou Y, Chen G, Long Z, Wang J (2014) RSC Adv 4:42092–42113
Nidheesh PV (2015) RSC Adv 5:40552–40577
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su CY (2014) Chem Soc Rev 43:6011–6061
Xue Z, Ma M-G, Li Z, Mu T (2016) RSC Adv 6:98874–98892
Santoro S, Kozhushkov SI, Ackermann L, Vaccaro L (2016) Green Chem 18:3471–3493
Khalafi-Nezhad A, Mohammadi S (2013) RSC Adv 3:4362–4371
Taheri M, Ghiaci M, Shchukarev A (2018) New J Chem 42:587–597
Sun J, Wang J, Cheng W, Zhang J, Li X, Zhang S, She Y (2012) Green Chem 14:654–660
Kaboudin B, Mostafalu R, Yokomatsu T (2013) Green Chem 15:2266–2274
Jean-Marie A, Griboval-Constant A, Khodakov AY, Monflier E, Diehl F (2011) Chem Commun 47:10767–10769
Salamatmanesh A, Miraki MK, Yazdani E, Heydari A (2018) Catal Lett 148:3257–3268
Wu J, Xu FZ, Feng SL, Xue W, Wang ZZ (2016) Heterocycles 92:1629–1642
Yadav GD, Kantam ML, Bhanage BM (2017) ACS Sustain Chem Eng 5:3597–3597
Urmode TD, Dawange MA, Shinde VS, Kusurkar RS (2017) Tetrahedron 73:4348–4354
Hapiot F, Monflier E (2017) Catalysts 7:173–184
Asghari S, Tajbakhsh M, Kenari BJ, Khaksar S (2011) Chin Chem Lett 22:127–130
Thombal RS, Jadhav AR, Jadhav VH (2015) RSC Adv 5:12981–12986
Wu J, Du X, Ma J, Zhang Y, Shi Q, Luo L, Song B, Yang S, Deyu H (2014) Green Chem 16:3210–3217
Girish YR, Sharath Kumar KS, Thimmaiah KN, Rangappa KS, Shashikanth S (2015) RSC Adv 5:75533–75546
Sabzi NE, Kiasat AR (2018) Catal Lett 148:2654–2664
Rai P, Srivastava M, Yadav S, Singh J, Singh J (2015) Catal Lett 145:2020–2028
Tayade YA, Patil DR, Wagh YB, Jangle AD, Dalal DS (2015) Tetthedron Lett 56:666–673
Patil DR, Ingole PG, Singh K, Dalal DS (2013) J Incl Phenom Macrocycl Chem 76:327–332
Tayade YA, Dalal DS (2017) Catal Lett 147:1411–1421
Sudhan PN, Ghashang M, Mansoor SS (2016) BJBAS 5:340–349
Gong K, Wang H, Ren X, Wang Y, Chen J (2015) Green Chem 17:3141–3147
Molnar A, Papp A (2014) Catal Sci Technol 4:295–310
Che F, Wang Y, Shen T, An X, Song Q (2015) C R Chimie 18:607–610
Gu Y (2012) Green Chem 14:2091–2128
Brahmachari G (2015) ACS Sustain Chem Eng 3:2058–2066
Shen T, Fu Z, Che F, Dang H, Lin Y, Song Q (2015) Tetrahedron Lett 56:1072–1075
Rigi F, Shaterian HR (2017) Polycycl Aromat Comp 37:314–326
Maleki A, Jafari AA, Yousefi S (2017) Carbohydr Polym 175:409–416
Bakherad M, Doosti R, Mirzaee M, Jadidi K (2017) IJC 7:27–35
Khalafi-Nezhad A, Shahidzadeh ES, Sarikhani S, Panahi F (2013) Tetrahedron Lett 379:1–8
Panda S, Roy A, Deka SJ, Trivedi V, Manna D (2016) ACS Med Chem Lett 7:1167–1172
Fu Z, Qian K, Li S, Shen T, Song Q (2016) Tetrahedron Lett 57:1104–1108
Wang SL, Ding J, Shi F, Liu YP, Jiang B, Ma N, Tu SJ (2012) J Heterocycl Chem 49:521
Kumar M, Sribalan R, Padmini V (2017) ChemistrySelect 2:489–493
Lakshmanan S, Ramalakshmi N (2016) Synth Commun 46:2045–2052
Li XT, Zhao AD, Mo LP, Zhang ZH (2014) RSC Adv 4:51580–51588
Sadjadi S, Heravi MM, Daraie M (2017) Res Chem Intermed 43:2201–2214
Bakherad M, Keivanloo A, Gholizadeh M, Doosti R, Javanmardi M (2017) Res Chem Intermed 43:1013–1029
Nasresfahani Z, Kassaee MZ (2017) ChemistrySelect 2:9642–9646
Tipale MR, Khillare LD, Deshmukh AR, Bhosale MR (2018) J Heterocycl Chem 00:00
Ziarani GM, Aleali F, Lashgari N, Badiei A, Soorkic AA (2018) IJPR 17:525–534
Heravi MM, Mousavizadeh F, Ghobadi N, Tajbakhsh M (2014) Tetrahedron Lett 55:1226–1228
Dastkhoon S, Tavakoli Z, Khodabakhshi S, Baghernejad M, Abbasabadi MK (2015) New J Chem 39:7268–7271
Ganesan A, Kothandapani J, Subramaniapillai SG (2016) RSC Adv 6:20582–20587
Patil A, Salunkhe R (2018) Res Chem Intermed 44:3337–3348
Lohar T, Kumbhar A, Patil A, Kamat S, Salunkhe R (2019) Res Chem Intermed 45:1639–1651
Mane AH, Patil AD, Kamat SR, Salunkhe RS (2018) ChemistrySelect 3:6454–6458
Patil A, Mane A, Kamat S, Lohar T, Salunkhe R (2019) Res Chem Intermed 45:3441–3452
Acknowledgements
The authors would like to thank Department of Chemistry, Shivaji University, Kolhapur for providing research facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, A., Gajare, S., Rashinkar, G. et al. β-CD-SO3H: Synthesis, Characterization and Its Application for the Synthesis of Benzylpyrazolyl Naphthoquinone and Pyrazolo Pyranopyrimidine Derivatives in Water. Catal Lett 150, 127–137 (2020). https://doi.org/10.1007/s10562-019-02928-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02928-y