Abstract
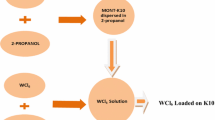
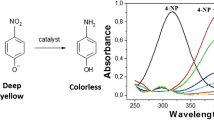
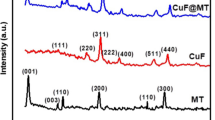
Cucurbit[6]uril (CB6) was used as a molecular glue for preparation of a new hybrid material using exfoliated montmorillonite clay and copper ferrite magnetic nanoparticles. Hybrid material was prepared by ultra-sonication method and characterised by FTIR, DR UV–Vis, XRD, FE-SEM, TEM, TGA and BET surface area measurements. The catalytic activity of the hybrid was examined for reduction of a potential industrial pollutant, 4-nitrophenol. The hybrid exhibited good catalytic activity (kapp 0.026 s−1) with low catalyst loading (∼0.05 mg/ml) and was found suitable for large scale application. The hybrid catalyst was successfully recycled and reused up to eight reaction cycles without any loss in catalytic activity. Here, CB6 acted like a molecular glue stabilising the hybrid catalyst. This greatly improved reusability in comparison with bare nanoparticles and clay composite. This approach where CB6 was used as a molecular glue can be conveniently utilised for stabilising nanoparticles on various solid support materials to develop better quality catalysts, composites and hybrid materials.
Graphic Abstract












Similar content being viewed by others
References
Shahwan T, Abu Sirriah S, Nairat M et al (2011) Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J 172:258–266
Astruc D, Lu F, Aranzaes JR (2005) Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew Chem-Int Ed 44:7852–7872
Cao M, Lin J, Yang H, Cao R (2010) Facile synthesis of palladium nanoparticles with high chemical activity using cucurbit [6] uril as protecting agent w. Chem Commun 46:5088–5090
Lu X, Masson E (2011) Formation and stabilization of silver nanoparticles with cucurbit[n]urils (n = 5-8) and cucurbituril-based pseudorotaxanes in aqueous medium. Langmuir 27:3051–3058
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—a review. Polym Sci 38:1231–1261
Benyettou F, Milosevic I, Lalatonne Y et al (2013) Toward theranostic nanoparticles: CB[7]-functionalized iron oxide for drug delivery and MRI. J Mater Chem B 1:5076–5082
Benyettou F, Nchimi- Nono K, Jouiad M et al (2015) Viologen-templated arrays of cucurbit[7]uril-modified iron-oxide nanoparticles. Chem A 21:4607–4613
Benyettou F, Motte L, Traboulsi H, Mazher J, Pasricha R, Olsen JC, Trabolsi A, Guenin E (2018) Palladium-loaded cucurbit [7] uril-modified iron oxide nanoparticles for C–C cross-coupling reactions. Chem Eur J 24:2349–2353
Qiu XL, Zhou Y, Jin XY et al (2015) One-pot solvothermal synthesis of biocompatible magnetic nanoparticles mediated by cucurbit[n]urils. J Mater Chem C 3:3517–3521
Qiao H, Jia J, Chen W et al (2018) Magnetic regulation of thermo-chemotherapy from a cucurbit[7]uril-crosslinked hybrid hydrogel. Adv Healthc Mater 8(2):1801458
Taylor R (2016) Precise subnanometer plasmonic junctions for precise subnanometer plasmonic junctions for SERS within gold nanoparticle assemblies using cucurbit [n] uril “glue”. ACS Nano 5(5):3878–3887
Mishra T, Parida K (1997) Transition-metal oxide pillared clays: part 2—a comparative study of textural and acidic properties of manganese(III) pillared montmorillonite and pillared acid-activated montmorillonite. J Mater Chem 7:147–152
Bagchi B, Thakur P, Kool A et al (2014) In situ synthesis of environmentally benign montmorillonite supported composites of Au/Ag nanoparticles and their catalytic activity in the reduction of p-nitrophenol. RSC Adv 4:61114–61123
Kloprogge JT (1999) Synthesis of smectite clay minerals: a critical review. Clays Clay Miner 47:529–554
Ganguly S, Dana K, Mukhopadhyay TK et al (2011) Organophilic nano clay: a comprehensive review. Trans Indian Ceram Soc 70:189–206
Kausar A, Iqbal M, Javed A et al (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407
Higson FK (1992) Microbial degradation of nitroaromatic compounds. Advances in applied microbiology. Acedemic Press, Inc., New York, pp 1–19
Gupta VK, Sharma S, Yadav IS, Mohan D (1998) Utilization of bagasse fly ash generated in the sugar industry for the removal and recovery of phenol andp-nitrophenol from wastewater. J Chem Technol Biotechnol 71:180–186
Di Paola A, Augugliaro V, Palmisano L et al (2003) Heterogeneous photocatalytic degradation of nitrophenols. J Photochem Photobiol, A 155:207–214
Feng J, Su L, Ma Y et al (2013) CuFe2O4 magnetic nanoparticles: a simple and efficient catalyst for the reduction of nitrophenol. Chem Eng J 221:16–24
Pradhan N, Pal A, Pal T (2002) Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf A 196:247–257
Devi TB, Ahmaruzzaman M (2017) Bio-inspired facile and green fabrication of Au@Ag@AgCl core–double shells nanoparticles and their potential applications for elimination of toxic emerging pollutants: a green and efficient approach for wastewater treatment. Chem Eng J 317:726–741
Cao E, Duan W, Wang F et al (2017) Natural cellulose fiber derived hollow-tubular-oriented polydopamine: in-situ formation of Ag nanoparticles for reduction of 4-nitrophenol. Carbohydr Polym 158:44–50
Seo YS, Ahn E-Y, Park J et al (2017) Catalytic reduction of 4-nitrophenol with gold nanoparticles synthesized by caffeic acid. Nanoscale Res Lett 12:7
Lebaschi S, Hekmati M, Veisi H (2017) Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J Colloid Interface Sci 485:223–231
You JG, Shanmugam C, Liu YW et al (2017) Boosting catalytic activity of metal nanoparticles for 4-nitrophenol reduction: modification of metal naoparticles with poly(diallyldimethylammonium chloride). J Hazard Mater 324:420–427
Goyal A, Bansal S, Singhal S (2014) Facile reduction of nitrophenols: comparative catalytic efficiency of MFe2O4 (M = Ni, Cu, Zn) nano ferrites. Int J Hydrog Energy 39:4895–4908
Zhang H, Gao S, Shang N et al (2014) Copper ferrite-graphene hybrid: a highly efficient magnetic catalyst for chemoselective reduction of nitroarenes. RSC Adv 4:31328–31332
Jansen K, Buschmann H, Wego A et al (2001) Solubility and amine complex formation. Solutions 39:357–363
Yoonessi M, Toghiani H, Kingery WL, Pittman CU (2004) Preparation, characterization, and properties of exfoliated/delaminated organically modified clay/dicyclopentadiene resin nanocomposites. Macromolecules 37:2511–2518
Zhang G, Qu J, Liu H et al (2007) CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere 68:1058–1066
Li M, Chen G (2013) Revisiting catalytic model reaction p-nitrophenol/NaBH4 using metallic nanoparticles coated on polymeric spheres. Nanoscale 5:11919
Bazgir A, Azimi SC (2013) Photocatalytic efficiency of CuFe2O4 by supporting on clinoptilolite in the decolorization of acid red 206 aqueous solutions. Iran J Catal 3:21–26
Wang Y, Li H, Zhang J et al (2016) Fe 3 O 4 and Au nanoparticles dispersed on the graphene support as a highly active catalyst toward the reduction of 4-nitrophenol. Phys Chem Chem Phys 18:615–623
Fang-hsin L, Doong R (2011) Bifunctional Au? Fe3O4 heterostructures for magnetically recyclable catalysis catalysis of nitrophenol reduction. J Phys Chem C 115:6591–6598
Noh J, Meijboom R (2014) Reduction of 4-nitrophenol as a model reaction for nanocatalysis. Appl Nanotechnol Water Res 9781118496:333–405
Kanagaraj M, Sathishkumar P, Selvan GK et al (2014) Structural and magnetic properties of CuFe2O4 as-prepared and thermally treated spinel nanoferrites. Indian J Pure Appl Phys 52:124–130
Mohan B, Park KH (2016) Superparamagnetic copper ferrite nanoparticles catalyzed aerobic, ligand-free, regioselective hydroboration of alkynes: influence of synergistic effect. Appl Catal A 519:78–84
Kefeni KK, Mamba BB, Msagati TAM (2017) Application of spinel ferrite nanoparticles in water and wastewater treatment: a review. Sep Purif Technol 188:399–422
Reddy DHK, Yun Y-S (2016) Spinel ferrite magnetic adsorbents: alternative future materials for water purification? Coord Chem Rev 315:90–111
Nakhate AV, Yadav GD (2017) Hydrothermal synthesis of CuFe2O4 magnetic nanoparticles as active and robust catalyst for N-arylation of indole and imidazole with aryl halide. ChemistrySelect 2:2395–2405
Chen G, Liu S, Chen S, Qi Z (2001) FTIR spectra, thermal properties, and dispersibility of a polysterene/montmorillonite nanocomposite. Macromol Chem Phys 202:1189–1193
Liu L, Zhao N, Scherman OA (2008) Ionic liquids as novel guests for cucurbit[6]uril in neutral water. Chem Commun 9:1070
Miao S, Liu Z, Han B et al (2006) Synthesis and characterization of TiO2–montmorillonite nanocomposites and their application for removal of methylene blue. J Mater Chem 16:579–584
Karunakaran C, SakthiRaadha S, Gomathisankar P, Vinayagamoorthy P (2013) Nanostructures and optical, electrical, magnetic, and photocatalytic properties of hydrothermally and sonochemically prepared CuFe2O4/SnO2. RSC Adv 3:16728
Hafeez HY, Lakhera SK, Karthik P et al (2018) Facile construction of ternary CuFe2O4-TiO2 nanocomposite supported reduced graphene oxide (rGO) photocatalysts for the efficient hydrogen production. Appl Surf Sci 449:772–779
Chang J, Ma J, Ma Q et al (2016) Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl Clay Sci 119:132–140
Xu J, Gao J, Wang W et al (2018) Noble metal-free NiCo nanoparticles supported on montmorillonite/MoS2 heterostructure as an efficient UV–visible light-driven photocatalyst for hydrogen evolution. Int J Hydrog Energy 43:1375–1385
Okamoto M, Nam PH, Maiti P et al (2001) A house of cards structure in polypropylene/clay nanocomposites under elongational flow. Nano Lett 1:295–298
Kelessidis VC (2017) Yield stress of bentonite dispersions. Rheol Open Access 1:1–12
Luo W, Fukumori T, Guo B et al (2017) Effects of grinding montmorillonite and illite on their modification by dioctadecyl dimethyl ammonium chloride and adsorption of perchlorate. Appl Clay Sci 146:325–333
Mandlimath TR, Gopal B (2011) Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-aminophenol. J Mol Catal A 350:9–15
Jin XY, Wang F, Cong H, Tao Z (2016) Host–guest interactions of hemicucurbiturils with aminophenols. J Incl Phenom Macrocycl Chem 86:241–248
Acknowledgements
This work was financially supported by Department of Science and Technology, Govt. of India (Grant No. EMR/2016/003186, DST/TM/WTI/WIC/2K17/100(G)) and Office of Research and Sponsored Projects, Pandit Deendayal Petroleum University (Grant No. ORSP/R&D/SRP/2017/NAPY & CKPY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trivedi, M.U., Patlolla, C.K., Misra, N.M. et al. Cucurbit[6]uril Glued Magnetic Clay Hybrid as a Catalyst for Nitrophenol Reduction. Catal Lett 149, 2355–2367 (2019). https://doi.org/10.1007/s10562-019-02853-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02853-0