Abstract
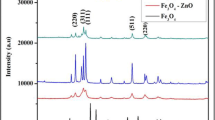
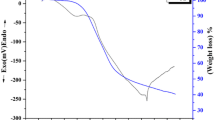
In recent years, magnesia stabilized zirconia (MgO–ZrO2) nanocomposite has made a versatile progress in applications due to its outstanding resistance to temperature and corrosion. It has been found to have dominant mechanical properties for industrial applications such as manufacturing of high temperature nozzles, thermal barrier coatings for gas turbine blades etc. Among all phases of zirconia, tetragonal and cubic are found to have versatile applications due to its stability using suitable application based dopants at room temperature. In the present work, magnesia–zirconia nanocomposites (MgO–ZrO2) have synthesized using sol–gel method at 400 and 600 °C. In addition to that the effect of citric acid and urea as the modifiers on nanocomposite has studied efficiently. The characterization was accomplished by X-ray diffraction, Scanning Electron Microscope with EDX, Raman, Absorbance Study and Fourier Transform Infra-Red Spectroscopy. MgO–ZrO2 nanocomposite was used as a heterogenous catalyst in the reduction of 4-nitrophenol and its antibacterial studies was determined. The crystallite size of the composite prepared using citric acid was found to be 4.5 nm and the band gap energy value was found to be 4.9 eV. The morphology of MgO–ZrO2 prepared using citric acid has exhibited flake like structure with smooth surface. The activity of nanocomposite was enhanced in the reduction of 4-nitrophenol owing to smaller crystallite size.
Graphical Abstract



















Similar content being viewed by others
References
Noguera C (1996) Phycics and chemistry at oxides surfaces. Cambridge University, Cambridge
Kung HH (1989) Transition metal oxides: surface chemistry and catalysis. Elsevier, Amsterdam
Zhang Q, Shen J, Wang J, Wu G, Chen L (2000) Sol-gel derived ZrO2-SiO2 highly reflective coatings. Int J Inorg Mater 2:319–323
Garvie RC, Hannink RH, Pascoe RT (1975) Ceramic steel. Nature 258:703–704
Subbarao EC, Heuer AH, Hobbs (1981) Zirconia-an overview. Adv Ceram Sci and Technol 3:311–324
Anpo M, Yamada Y, Kubokawa Y, Coluccia S, Zecchina A, Che M (1988) Photoluminescence properties of MgO powders with coordinatively unsaturated surface ions. Faraday Trans 84:751–764
Murphy DM, Farley RD, Purnell IJ, Rowlands CC, Yacob AR, Paganini M, Giamello E (1999) Surface defect sites formed on partially and fully dehydrated MgO: an EPR/ENDOR study. J Phys Chem B 103:1944–1953
Richards R, Li W, Decker F, Davidson S, Koper C, Zaikovski O, Volodin V, Rieker A, Klabunde T (2000) Consolidation of metal oxide nanocrystals. J Am Chem Soc 122:4921–4925
Cypres R, Wollast R (1963) Contribution on the polymorphic conversion of pure zirconia. J Raucq Chem Ber 40:527–531
Vijayakumara M, Selvasekarapandiana S, Bhuvaneswaria MS, HiranKumara G, Ramprasad G, Subramanian R, Angelo PC (2003) Synthesis and ion dynamics studies of nanocrystalline Mg stabilized zirconia. Physica 334:390–397
Henrique Pedro, Camargo Cury, Satyanarayana Kestur Gundappa, Wypych Fernando (2009) Nanocomposites: synthesis, structure, properties and new application opportunities. Mater Res 12(1–39):11
Leon C, Luci ML, Santamaria J (1997) Correlated ion hopping in single-crystal yttria-stabilized zirconia. J Phy Rev 55(882–887):12
Taylor R, Brandon JR, Morrell Paul (1992) Microstructure, composition and property relationships of plasma-sprayed thermal barrier coatings. J Surf Coat Tech 50(141–149):13
Mucillio E, Roeha R, Muccilo R (2002) Preparation of Gd2O3-doped ZrO2 by polymeric precursor techniques. Mater Lett 53(353–358):14
Patil KC, Aruna ST, Ekambaram S (1997) Combustion synthesis. Solid State Mater Sci 2(158–165):15
Mohamed G (2012) Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. J Ind Tex 41:222–240
Tian Xike, Zeng Yanling, Xiao Ting, Yang Chao, Wang Yanxin, Zhang Suxin (2011) Fabrication and stabilization of nanocrystalline ordered mesoporous MgO–ZrO2 solid solution. Micro Meso Mater 143(357–361):16
Saavedra MJ, Parada C, Figueiredo MO, Correa dos Santos A (1993) Ca-Zr-O system: synthesis and characterization of compounds using several routes of preparation. Solid State Ion 63:213–217
Soja KV, Chandramouli V, ShabanaKhan Clinsha PC, Anthonysamy S (2014) Microwave assisted gel-combustion synthesis of 8 mol% YSZ: a study of the effect of fuel on the ionic conductivity. Ceram Int 40:16689–16699
Hajizadeh-Oghaz Morteza, Razavi Reza Shoja, Khajelakzay Mohammad (2015) Optimizing sol-gel synthesis of magnesia-stabilized zirconia (MSZ) nanoparticles using Taguchi robust design for thermal barrier coatings (TBCs) applications. J Sol-Gel Sci Technol 73:227–241
Cullity BD (1956) Elements of X-ray diffraction. Addison-Wesley Inc., Massachusetts
Frison R, Heiroth S, Rupp JLM, Conder K, Barthazy EJ, Muller E, Horisberger M, Dobeli M, Gauckler LJ (2013) Crystallization of 8 mol % yttria-stabilized zirconia thin-films deposited by RF-sputtering. Solid State Ionics 232:29–36
Combemale Lionel, Caboche Gilles, Stuerga Didier, Chaumont Denis (2005) Microwave synthesis of yttria stabilized zirconia. Mater Res Bull 40:529–536
Rajeswari K, Buchi Suresh M, Hareesh US, Srinivasa RY, Das Dibakar, Johnson Roy (2011) Studies on ionic conductivity of stabilized zirconia ceramics (8YSZ) densified through conventional and non-conventional sintering methodologies. Ceram Int 37:3557–3564
Sadat-Shojai M, Khorasani MT, Jamshidi A (2012) Hydrothermal processing of hydroxyapatite nanoparticles-A Taguchi experimental design approach. J Cryst Growth 361:73–84
Norouzbeigi R, Edrissi M (2011) Modification and optimization of nano-crystalline Al2O3combustion synthesis using Taguchi L16 array. Mater Res Bull 46:1615–1624
Lopez Enrique Fernandez, Escribano Vicente Sanchez, Panizza Marta, Maria MC (2001) Vibrational and electronic spectroscopic properties of zirconia powders. J Mater Chem 11:1891–1897
Renuka L, Anantharaju KS, Sharma SC, Nagaswarupa HP, Prashantha SC, Nagabhushana H, Vidya YS (2016) J Alloy Compd 5:609–622
Bansal Pratibha, Chaudhary Ganga Ram, Mehta SK (2015) Comparative study of catalytic activity of ZrO2 nanoparticles for sonocatalytic and photocatalytic degradation of cationic and anionic dyes. Chem Eng Journ 280:475–485
Manivasakan P, Rajendran V, Rauta PR, Sahu BB, Panda BK (2011) Synthesis of monoclinic and cubic ZrO2 nanoparticles from zircon. J Am Ceram Soc 94:1410–1420
Panigrahi S, Basu S, Praharaj S, Pande S, Jana S, Pal A, Ghosh SK, Pal T (2007) Synthesis and Size-Selective Catalysis by Supported Gold Nanoparticles: study on Heterogeneous and Homogeneous Catalytic Process. J Phys Chem C 111:4596–4605
Mohamed G (2012) Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. J Ind Tex 41:222–240
Jangra SL, Stalin K, Dilbaghi N, Kumar S, Tawale J, Singh SP, Pasricha R (2012) Antimicrobial activity of zirconia (ZrO2) nanoparticles and zirconium complexes. J Nanosci Nanotec 12:7105–7112
Thakare VG, Joshi PA, Godse RR, Bhatkar VB, Wadegaokar PA, Omanwar SK (2016) Evaluation of biological activities of nanocrystalline tetragonal zirconia synthesized via sol-gel method. Int J Pharm Pharm Sci 8:125–131
Narayanan K, Sakthivel C, Prabha I (2019) MgO–ZrO2 mixed nanocomposites: fabrication methods and applications. Mater Today Sustain. https://doi.org/10.1016/j.mtsust.2019.100007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for each contributing author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keerthana, L., Sakthivel, C. & Prabha, I. Influence of Citric Acid/Urea on High Performance Magnesia–Zirconia Nanocomposite: Synthesis, Characterization and Catalytic Approaches. Catal Lett 149, 2683–2695 (2019). https://doi.org/10.1007/s10562-019-02824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02824-5