Abstract
Cage like CuFe2O4 hollow nanostructure has been synthesized successfully using hard template method under the hydrothermal condition. Cu(NO3)2·6H2O, Fe(NO3)2·9H2O and glucose were dissolved in water, and the mixture was heated to 180 °C in an autoclave. The removal of carbon was achieved by calcination at 800 °C and finally, the cage like CuFe2O4 hollow structure was obtained. This cage like CuFe2O4 hollow structure was characterized by FE-SEM, EDS, TEM and XRD. The catalytic performance of this hollow nanostructure was evaluated for the synthesis of bis pyrazol-5-ols. To this end, the one pot condensation reactions of phenylhydrazine, ethyl acetoacetate and different aromatic aldehyde at 80 °C under the solvent free condition were performed. The optimum amount of applied catalyst for this transformation was obtained to be 0.04 mol %. Noteworthy, catalyst was easily recoverable and was reused for 7 times with the remaining of its initial structure as well as its catalytic activity.
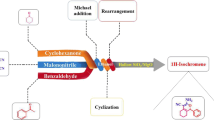
Graphical Abstract
Cage like CuFe2O4 hollow nanostructure are stably and efficiently attainable for conversion of precursors to 4,4′-(aryl methylene)bis(3-methyl-1H-pyrazol-5-ol)s








Similar content being viewed by others
References
Tangcharoen T, Klysubun W, Kongmark C, Pecharapa W (2014) Phys Status Solidi A 211:1903
Gimenes R, Baldissera MD, Da Silva MR, Da Silveira CA, Soares DA, Perazolli LA, Da Silva MR, Zaghete MA (2012) Ceram Int 38:741
Prabhakaran T, Hemalatha J (2011) J Alloys Compd 509:7071
Azizi A, Sadrnezhaad SK (2010) Ceram Int 36:2241
Si C, Zhang Y, Zhang C, Gao H, Ma W, Lv L, Zhang Z (2017) Electrochim Acta 245:829
Zeng H, Rice PM, Wang SX, Sun S (2004) J Am Chem Soc 126:11458
Qian HS, Hu Y, Li ZQ, Yang XY, Li LC, Zhang XT, Xu R (2010) J Phys Chem C 114:17455
Du N, Xu Y, Zhang H, Zhai C, Yang D (2010) Nanoscale Res Lett 5:1295
Knez M, Scholz R, Nielsch K, Pippel E, Hesse D, Zacharias M, Gösele U (2006) Nat Mater 5:627
Prieto G, Tüysüz H, Duyckaerts N, Knossalla J, Wang GH, Schüth F (2016) Chem Rev 116:14056
Innocenzi P, Martucci A, Guglielmi M, Bearzotti A, Traversa E (2001) Sens Actuators B Chem 76:299
Choi HJ, Cho MS, Kang KK, Ahn WS (2000) Microporous Mesoporous Mater 39:19
Bach U, Lupo D, Comte P, Moser JE, Weissörtel F, Salbeck J, Spreitzer H, Grätzel M (1998) Nature 1395:583
Grätzel M (2001) Pure Appl Chem 73:459
Chen QW, Bahnemann DW (2000) J Am Chem Soc 122:970
McDonald E, Jones K, Brough PA, Drysdale MJ, Workman P (2006) Curr Top Med Chem 16:1193
Perez-Fernández R, Goya P, Elguero J (2014) ARKIVOC (ii) 233
Wiley RH, Behr LC (1967) Pyrazoles, pyrazolines, pyrazolidines, indazoles and condensed ring. John Wiley & sons, New Jersey
Sugiura S, Ohno S, Ohtani O, Izumi K, Kitamikado T, Asai H, Kato K, Hori M, Fujimura H (1977) J Med Chem 20:80
Prakash O, Kumar R, Parkash V (2008) Eur J Med Chem 43:435
Antre RV, Cendilkumar A, Nagarajan RR, Goli D, Oswal RJ (2012) J Sci Res 4:183
Kappe CO (2000) Eur J Med Chem 35:1043
Hasaninejad A, Shekouhy M, Zare A, Ghattali SH, Golzar N (2011) J Iran Chem Soc 8:411
Singh D, Singh D (1984) J Chem Eng Data 29:355
Shi DQ, Chen J, Wu N, Zhuang QY, Wang XS (2005) Chin J Org Chem 25:405
Karimi-Jaberi Z, Pooladian B, Moradi M, Ghasemi E (2012) Chin J Catal 33:1945
Gouda MA, Abu-Hashem AA (2012) Green Chem Lett Rev 5:203
Sujatha K, Shanthi G, Selvam NP, Manoharan S, Perumal PT, Rajendran M (2009) Bioorg Med Chem Lett 19:4501
Baghernejad M, Niknam K (2012) Int J Chem 4:52
Niknam K, Saberi D, Sadegheyan M, Deris A (2010) Tetrahedron Lett 51:692
Zarghani M, Akhlaghinia B (2015) RSC Adv 5:87769
Jahanshahi R, Akhlaghinia B (2017) Chem Pap 71:1351
Safaiee M, Zolfigol MA, Derakhshan-Panah F, Khakyzadeh V, Mohammadi L (2016) Croat Chem Acta 89:317
Zolfigol MA, Ayazi-Nasrabadi R, Baghery S (2015) RSC Adv 5:71942
Chen Z, Cui ZM, Niu F, Jiang L, Song WG (2010) Chem Commun 46:6524
Li Y, Zhou P, Dai Z, Hu Z, Sun P, Bao J (2006) New J Chem 30:832
Ikeda S, Ishino S, Harada T, Okamoto N, Sakata T, Mori H, Kuwabata S, Torimoto T, Matsumura M (2006) Angew Chem Int Ed 45:7063
Rajabzadeh M, Eshghi H, Khalifeh R, Bakavoli M (2018) Appl Organomet Chem 32:e4052
Rajabzadeh M, Eshghi H, Khalifeh R, Bakavoli M (2016) RSC Adv 6(23):19331–19340
Rajabzadeh M, Eshghi H, Khalifeh R, Bakavoli M (2017) Appl Organomet Chem 31:e3647
Sharghi H, Jokar M, Doroodmand MM, Khalifeh R (2010) Adv Synth Catal 352:3031
Sharghi H, Khalifeh R (2007) Heterocycles 71:1601
Sharghi H, Khalifeh R (2008) Can J Chem 86:426
Khalifeh R, Sharghi H, Rashidi Z (2013) Heteroatom Chem 24:372
Khalifeh R, Ghamari M (2016) J Braz Chem Soc 27:759
Sharghi H, Khalifeh R, Mansouri SG, Aberi M, Eskandari MM (2011) Catal Lett 141:1845
Rajabzadeh M, Khalifeh R, Eshghi H, Sorouri M (2019) Catal Lett 149:1125
Khalifeh R, Karimzadeh F (2019) Can J Chem 97:303
Rezaei F, Amrollahi MA, Khalifeh R (2019) Inorganica Chim Acta 489:8
Sun X, Li Y (2004) Angew Chem Int Ed 43:597
Wang W, Wang SX, Qin XY, Li JT (2005) Synth Commun 35:1263
Hasaninejed A, Kazerooni MR, Zare A (2013) ACS Sustain Chem Eng 1:679
Zhou Z, Zhang Y (2014) Green Chem Lett Rev 7:18
Niknam K, Mirzaee S (2011) Synth Commun 41:2403
Tayebi S, Niknam K (2012) Iran J Catal 2:69
Acknowledgement
We gratefully acknowledge the support of this work by the Shiraz University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khalifeh, R., Shahimoridi, R. & Rajabzadeh, M. Design and Synthesis of Novel Cage like CuFe2O4 Hollow Nanostructure as an Efficient Catalyst for Synthesis of 4,4′-(aryl methylene)bis(3-methyl-1H-pyrazol-5-ol)s. Catal Lett 149, 2864–2872 (2019). https://doi.org/10.1007/s10562-019-02818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02818-3