Abstract
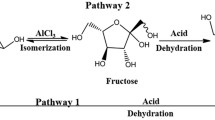
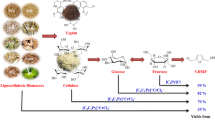
In this work, an environment-friendly and recyclable system was developed for converting waxy corn starch to HMF, with boric acid and choline chloride acting as co-catalysts, and a biphasic medium of water-MIBK or water-THF used as solvent. A central composite design based Response Surface Methodology was used to optimise the reaction parameters. The highest HMF yield obtained for water-MIBK was 35.9 mol%, while for water-THF, a maximum yield of 60.3 mol% was attained. However, the water-MIBK system was more recyclable, with minimal decrease in HMF yields observed even after ten rounds of reuse. Therefore, this system merits further investigation with other feedstock and on a larger scale.
Graphical Abstract








Similar content being viewed by others
References
Cherubini F (2010) The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energ Convers Manage 51(7):1412–1421
Mukherjee A, Dumont M-J, Review RV (2015) Sustainable production of hydroxymethylfurfural and levulinic acid: challenges and opportunities. Biomass Bioenergy 72:143–183
Pagán-Torres YJ, Wang T, Gallo JMR et al (2012) Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Brønsted acid catalysts in water in a biphasic reactor with an alkyl phenol solvent. ACS Catal 2(6):930–934
Liu F, Audemar M, De Oliveira Vigier K et al. (2013) Selectivity enhancement in the aqueous acid-catalyzed conversion of glucose to 5-hydroxymethylfurfural induced by choline chloride. Green Chem 15(11):3205–3213
Zhang Z, Wang Q, Xie H et al (2011) Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural by germanium(IV) chloride in ionic liquids. ChemSusChem 4(1):131–138
Hu L, Sun Y, Lin L (2012) Efficient conversion of glucose into 5-hydroxymethylfurfural by chromium(III) chloride in inexpensive ionic liquid. Ind Eng Chem Res 51(3):1099–1104
De S, Dutta S, Saha B (2011) Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride catalyst in water. Green Chem 13(10):2859
Roy Goswami S, Dumont M-J, Raghavan V (2016) Microwave assisted synthesis of 5-hydroxymethylfurfural from starch in AlCl3·6H2O/DMSO/[BMIM]Cl system. Ind Eng Chem Res 55(16):4473–4481
Tong X, Wang Y, Nie G et al (2015) Selective dehydration of fructose and sucrose to 5-hydroxymethyl-2-furfural with heterogeneous ge(IV) catalysts. Environ Prog Sustain Energy 34(1):207–210
Degirmenci V, Hensen EJM (2014) Development of a heterogeneous catalyst for lignocellulosic biomass conversion: glucose dehydration by metal chlorides in a silica-supported ionic liquid layer. Environ Prog Sustain Energy 33(2):657–662
Osatiashtiani A, Lee AF, Brown DR et al (2014) Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose. Catal Sci Technol 4(2):333–342
Martínez JJ, Silva DF, Aguilera EX et al (2017) Dehydration of glucose to 5-hydroxymethylfurfural using LaOCl/Nb2O5 catalysts in hot compressed water conditions. Catal Lett 147(7):1765–1774
Gromov NV, Taran OP, Semeykina VS et al (2017) Solid acidic NbOx/ZrO2 catalysts for transformation of cellulose to glucose and 5-hydroxymethylfurfural in pure hot water. Catal Lett 147(6):1485–1495
Walia M, Sharma U, Agnihotri VK et al (2014) Silica-supported boric acid assisted conversion of mono- and poly-saccharides to 5-hydroxymethylfurfural in ionic liquid. RSC Adv 4(28):14414
Stahlberg T, Rodriguez-Rodriguez S, Fristrup P et al (2011) Metal-free dehydration of glucose to 5-(hydroxymethyl)furfural in ionic liquids with boric acid as a promoter. Chem Eur J 17(5):1456–1464
Román-Leshkov Y, Dumesic JA (2009) Solvent effects on fructose dehydration to 5-hydroxymethylfurfural in biphasic systems saturated with inorganic salts. Top Catal 52(3):297–303
Benoit M, Brissonnet Y, Guelou E et al (2010) Acid-catalyzed dehydration of fructose and inulin with glycerol or glycerol carbonate as renewably sourced co-solvent. ChemSusChem 3(11):1304–1309
Hansen TS, Mielby J, Riisager A (2011) Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem 13(1):109
McNeff CV, Nowlan DT, McNeff LC et al (2010) Continuous production of 5-hydroxymethylfurfural from simple and complex carbohydrates. Appl Catal A 384(1–2):65–69
Tong X, Yu L, Nie G et al (2015) Antimony-meditated efficient conversion of carbohydrates to 5-hydroxymethylfurfural in a simple THF-H2O binary solvent. Environ Prog Sustain Energy 34(4):1136–1141
Mukherjee A, Dumont M-J (2016) Influence of the starch structure in the synthesis and the yield of levulinic acid. Starch - Stärke 68:943–952
Mukherjee A, Dumont M-J (2016) Levulinic acid production from starch using microwave and oil bath heating: a kinetic modeling approach. Ind Eng Chem Res 55(33):8941–8949
Araujo PW, Brereton RG (1996) Experimental design I. Screening. Trends Anal Chem 15(1):26–31
AN55 Analytical Methods Committee (2013) Experimental design and optimisation (4) Plackett–Burman designs. Anal Methods 5(8):1901
Roy Goswami S, Mukherjee A, Dumont M-J et al (2016) One-pot conversion of corn starch into 5-hydroxymethylfurfural in water-[Bmim]Cl/MIBK biphasic media. Energy Fuel 30(10):8349–8356
Liu F, Sivoththaman S, Tan Z (2014) Solvent extraction of 5-HMF from simulated hydrothermal conversion product. Sustain Environ Res 24(2):149–157
Blumenthal LC, Jens CM, Ulbrich J et al (2016) Systematic identification of solvents optimal for the extraction of 5-hydroxymethylfurfural from aqueous reactive solutions. ACS Sustain Chem Eng 4(1):228–235
Weingarten R, Cho J, Xing R et al (2012) Kinetics and reaction engineering of levulinic acid production from aqueous glucose solutions. ChemSusChem 5(7):1280–1290
McKibbins WS (1962) Kinetics of the acid catalyzed conversion of glucose to 5-hydroxymethyl-2-furaldehyde and levulinic acid. Forest Prod J 12:17–23
Heimlich KR, Martin AN (1960) A kinetic study of glucose degradation in acid solution. J Am Pharm Assoc 49(9):592–597
Baugh KD, McCarty PL (1988) Thermochemical pretreatment of lignocellulose to enhance methane fermentation: I. Monosaccharide and furfurals hydrothermal decomposition and product formation rates. Biotechnol Bioeng 31(1):50–61
Paiva A, Craveiro R, Aroso I et al (2014) Natural deep eutectic solvents—solvents for the 21st century. ACS Sustain Chem Eng 2(5):1063–1071
Shekaari H, Zafarani-Moattar MT, Shayanfar A et al (2018) Effect of choline chloride/ethylene glycol or glycerol as deep eutectic solvents on the solubility and thermodynamic properties of acetaminophen. J Mol Liq 249:1222–1235
Azizi N, Dezfooli S, Khajeh M et al (2013) Efficient deep eutectic solvents catalyzed synthesis of pyran and benzopyran derivatives. J Mol Liq 186:76–80
Wu Z, Huang RR, Yu H et al (2017) Deep eutectic solvent synthesis of LiMnPO(4)/C nanorods as a cathode material for lithium ion batteries. Materials 10(2):134
Wagle DV, Deakyne CA, Baker GA (2016) Quantum chemical insight into the interactions and thermodynamics present in choline chloride based deep eutectic solvents. J Phys Chem B 120(27):6739–6746
Hiltunen J, Kuutti L, Rovio S et al (2016) Using a low melting solvent mixture to extract value from wood biomass. Sci Rep 6:32420
Locas CP, Yaylayan VA (2008) Isotope labeling studies on the formation of 5-(Hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J Agric Food Chem 56(15):6717–6723
Qian X (2012) Mechanisms and energetics for Brønsted acid-catalyzed glucose condensation, dehydration and isomerization reactions. Top Catal 55(3–4):218–226
Binder JB, Cefali AV, Blank JJ et al (2010) Mechanistic insights on the conversion of sugars into 5-hydroxymethylfurfural. Energy Environ Sci 3(6):765
Noma R, Nakajima K, Kamata K et al (2015) Formation of 5-(hydroxymethyl)furfural by stepwise dehydration over TiO2 with water-tolerant lewis acid sites. J Phys Chem C 119(30):17117–17125
Amiri H, Karimi K, Roodpeyma S (2010) Production of furans from rice straw by single-phase and biphasic systems. Carbohydr Res 345(15):2133–2138
Acknowledgements
The financial support of McGill University, the National Science and Engineering Research Council of Canada (NSERC), and the Programme de bourses d’excellence pour étudiants étrangers (PBEEE) scholarship of the Fonds de Recherche du Québec-Nature et technologies (FRQNT) is gratefully acknowledged. We are grateful to Dr. Vijaya Raghavan for permitting the use of his laboratory equipment. We are also immensely thankful to Mr Yvan Gariépy for his support in our work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mukherjee, A., Dumont, MJ. & Cherestes, A. Production of 5-Hydroxymethylfurfural from Starch Through an Environmentally-Friendly Synthesis Pathway. Catal Lett 149, 283–291 (2019). https://doi.org/10.1007/s10562-018-2597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2597-8