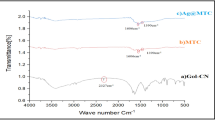
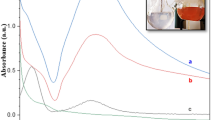
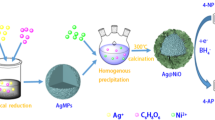
Abstract
The use of metal immobilized/decorated nanocomposites as catalyst were usually used in environmental pollution remediation and protection, industrial production, and biomedical applications. Finding a new and efficient method for the green synthesis of metal nanoparticles immobilized over porous material is of great interest. Synthesis of more stable and outstanding Cu@ZnO and Ag@ZnO nanocomposite for nitro aromatic compound reduction were reported in this work. The metal nanoparticles and nanocomposite was characterized using UV–Vis spectrum, XRD, Raman spectra, TEM, SAED, EDS, and FTIR techniques. The immobilized Cu and Ag nanoparticles are with an average size of 18 and 12 nm on ZnO surface respectively. Comparatively, the Cu/ZnO and Ag/ZnO nanocomposite acted as an efficient heterostructure catalyst in the reduction of p-nitrophenol to p-aminophenol than pure Cu and Ag nanoparticles with more stability up to six cycles. The characterization results inferred the synergic effect between metal and porous material played important role in its activity and stability of Cu@ZnO and Ag@ZnO nanocomposite more than pure Cu and Ag nanoparticles. It is proposed that Cu and Ag immobilized ZnO applicable in various catalytic activities were achieved.
Graphical Abstract
















Similar content being viewed by others
References
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34:257–264
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677
Suman TY, Rajasree SR, Jayaseelan C, Mary RR, Gayathri S, Aranganathan L, Remya RR (2016) GC–MS analysis of bioactive components and biosynthesis of silver nanoparticles using Hybanthus enneaspermus at room temperature evaluation of their stability and its larvicidal activity. Environ Sci Pollut Res 23:2705–2714
Murphy CJ, Gole AM, Hunyadi SE, Stone JW, Sisco PN, Alkilany A, Kinard BE, Hankins P (2008) Chemical sensing and imaging with metallic nanorods. Chem Commun 5:544–557
Saran S, Kamalraj G, Arunkumar P, Devipriya SP (2016) Pilot scale thin film plate reactors for the photocatalytic treatment of sugar refinery wastewater. Environ Sci Pollut Res 23:7730–17741
Padilla RH, Priecel P, Lin M, Lopez-Sanchez JA, Zhong Z (2017) A versatile sonication-assisted deposition–reduction method for preparing supported metal catalysts for catalytic applications. Ultrason Sonochem 35:631–639
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Bordbar M, Mortazavimanesh N (2016) Green synthesis of Pd/walnut shell nanocomposite using Equisetum arvense L. leaf extract and its application for the reduction of 4-nitrophenol and organic dyes in a very short time. Environ Sci Pollut Res 1–12
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res 34:181–190
Wang MQ, Ye C, Bao SJ, Zhang Y, Xu MW, Li Z (2016) Bimetal–organic-frameworks-derived yolk–shell-structured porous Co2P/ZnO@ PC/CNTs hybrids for highly sensitive non-enzymatic detection of superoxide anion released from living cells. Chem Commun 52:12442–12445
Sheng Q, Shen Y, Zhang J, Zheng J (2017) Ni doped Ag@ C core–shell nanomaterials and their application in electrochemical H2O2 sensing. Anal Methods 9:163–169
Goswami A, Rathi AK, Aparicio C, Tomanec O, Petr M, Pocklanova R, Gawande MB, Varma RS, Zboril R (2017) In situ generation of Pd–Pt core–shell nanoparticles on reduced graphene oxide (Pd@Pt/rGO) using microwaves: applications in dehalogenation reactions and reduction of olefins. ACS Appl Mater Interfaces 9:2815–2824
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D’Alessio M, Zambonin PG, Traversa E (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 17:5255–5262
Zhang Z, Zhang L, Wang S, Chen W, Lei Y (2001) A convenient route to polyacrylonitrile/silver nanoparticle composite by simultaneous polymerization–reduction approach. Polymer 42:8315–8318
Paul K, Gary AM (2014) Comprehensive organic synthesis, 2nd edn. Elsevier, New York
Udom I, Zhang Y, Ram MK, Stefanakos EK, Hepp AF, Elzein R, Schlaf R, Goswami DY (2014) A simple photolytic reactor employing Ag-doped ZnO nanowires for water purification. Thin Solid Films 564:258–263
Mori K, Miyawaki K, Yamashita H (2016) Ru and Ru–Ni nanoparticles on TiO2 support as extremely active catalysts for hydrogen production from ammonia–borane. ACS Catal 6:3128–3135
Nasrollahzadeh M, Sajadi M (2016) Preparation of Pd/Fe3O4 nanoparticles by use of Euphorbia stracheyi Boiss root extract: a magnetically recoverable catalyst for one-pot reductive amination of aldehydes at room temperature. J Colloid Interface Sci 464:147–152
Nasrollahzadeh M, Sajadi SM, Rostami-Vartooni A, Alizadeh M, Bagherzadeh M (2016) Green synthesis of the Pd nanoparticles supported on reduced graphene oxide using barberry fruit extract and its application as a recyclable and heterogeneous catalyst for the reduction of nitroarenes. J Colloid Interface Sci 466:360–368
Pudukudy M, Yaakob Z, Mazuki MZ, Takriff MS, Jahaya SS (2017) One-pot sol–gel synthesis of MgO nanoparticles supported nickel and iron catalysts for undiluted methane decomposition into COx free hydrogen and nanocarbon. Appl Catal B 218:298–316
Ozgür U, Alivov YI, Liu C, Teke A, Reshchikov M, Doğan S, Avrutin VCSJ., Cho SJ, Morkoc H (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:11–16
Zheng Y, Zheng L, Zhan Y, Lin X, Zheng Q, Wei K (2007) Ag/ZnO heterostructure nanocrystals: synthesis, characterization, and photocatalysis. Inorg Chem 46:6980–6986
Gao S, Jia X, Yang S, Li Z, Jiang K (2011) Hierarchical Ag/ZnO micro/nanostructure: green synthesis and enhanced photocatalytic performance. J Solid State Chem 184:764–769
Raja Rajeswari N, RamaLakshmi S, Muthuchelian K (2011) GC–MS analysis of bioactive components from the ethanolic leaf extract of Canthium dicoccum (Gaertn.) Teijsm & Binn. J Chem Pharm Res 3:792–798
Khare CP (2007) Indian medicinal plants: an illustrated dictionary. Springer Science Business Media, LLC, New York, p 393
Manjari G, Saran S, Rao AVB, Devipriya SP (2017) Phytochemical screening of Aglaia elaeagnoidea and their efficacy on antioxidant and antimicrobial growth. Int J Ayur Pharm Res 5:7–14
Ghosh S, Goudar VS, Padmalekha KG, Bhat SV, Indi SS, Vasan HN (2012) ZnO/Ag nanohybrid: synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv 2:930–940
Harish S, Archana J, Sabarinathan M, Navaneethan M, Nisha KD, Ponnusamy S, Muthamizhchelvan C, Ikeda H, Aswal DK, Hayakawa Y (2016) Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl Surf Sci 418:103–112
Ang W, Li X, Li S, Yan-Jun L, Wei-Wei L (2013) CuO nanoparticle modified ZnO nanorods with improved photocatalytic activity. Chin Phys Lett 30:046198–046202
Udom B, Pal PK (2011) Giri defect mediated magnetic interaction and high Tc ferromagnetism in Co doped ZnO nanoparticles. J Nanosci Nanotechnol 11:9167–9174
Lupan O, Chow L, Ono LK, Cuenya BR, Chai G, Khallaf H, Park S, Schulte A (2010) Synthesis and characterization of Ag-or Sb-doped ZnO nanorods by a facile hydrothermal route. J Phys Chem C 114:12401–12408
Kuriakose S, Satpati B, Mohapatra S (2014) Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys Chem Chem Phys 16(25):12741–12749
Soundarrajan P, Sankarasubramanian K, Sampath M, Logu T, Sethuraman K, Ramamurthi K (2015) Cu ions induced reorientation of crystallite in ZnO nano/micro rod arrays thin films. Phys E 71:56–63
Shankar SS, Ahmad A, Sastry M (2003) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotech Prog 19:1627–1631
Manjari G, Saran S, Arun T, Rao AVB, Devipriya SP (2017) Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. J Saudi Chem Soc 21:610–618
Saha S, Pal A, Kundu S, Basu S, Pal T (2009) Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 26:2885–2893
Rode CV, Vaidya MJ, Chaudhari RV (1999) Synthesis of p-aminophenol by catalytic hydrogenation of nitrobenzene. Org Process Res Dev 3:465–470
Phan NT, Van Der Sluys M, Jones CW (2006) On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings–homogeneous or heterogeneous catalysis, a critical review. Adv Syn Cat 348:609–679
Gangula A, Podila R, Karanam L, Janardhana C, Rao AM (2011) Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir 27:15268–15274
Nasrollahzadeh M, Sajadi SM (2015) Green synthesis of copper nanoparticles using Ginkgo biloba L. leaf extract and their catalytic activity for the Huisgen [3 + 2] cycloaddition of azides and alkynes at room temperature. J Colloid Interface Sci 1:141–147
Choi Y, Bae HS, Seo E, Jang S, Park KH, Kim BS (2011) Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J Mater Chem 21:15431–15436
Dhakshinamoorthy A, Asiri AM, Garcia H (2015) Metal–organic frameworks catalyzed C–C and C–heteroatom coupling reactions. Chem Soci Rev 44:1922–1947
Yao T, Zuo Q, Wang H, Wu J, Xin B, Cui F, Cui T (2015) A simple way to prepare Pd/Fe3O4/polypyrrole hollow capsules and their applications in catalysis. J Colloid Interface Sci 450:366–373
Momeni SS, Nasrollahzadeh M, Rustaiyan A (2016) Green synthesis of the Cu/ZnO nanoparticles mediated by Euphorbia prolifera leaf extract and investigation of their catalytic activity. J Colloid Interface Sci 472:173–179
Zhou Y, Fang C, Fang Y, Zhu F, Liu H, Ge H (2016) Hydrogen generation mechanism of BH4– spontaneous hydrolysis: a sight from ab initio calculation. Int J Hydrog Energy 41:22668–22676
Acknowledgements
The authors are grateful to Pondicherry University for providing fellowship for the first two authors. The authors are acknowledge to STIC, Cochin and Central instrumentation facility, Pondicherry University for characterization analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjari, G., Saran, S., Devipriya, S.P. et al. Novel Synthesis of Cu@ZnO and Ag@ZnO Nanocomposite via Green Method: A Comparative Study for Ultra-Rapid Catalytic and Recyclable Effects. Catal Lett 148, 2561–2571 (2018). https://doi.org/10.1007/s10562-018-2435-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2435-z