Abstract
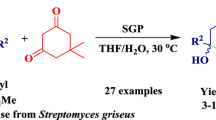
The direct asymmetric Michael addition of malonates and enones was promoted by protease from Streptomyces griseus for the first time. Yields of up to 84% with enantioselectivities of up to 98% enantiomeric excess (ee) were achieved under optimized conditions.
Graphical Abstract
Protease from Streptomyces griseus (SGP) was used for the first time as a biocatalyst in asymmetric Michael reaction of malonates.


Similar content being viewed by others
References
Sibi MP, Manyem S (2000) Tetrahedron 56:8033–8061
Krause N, Hoffmann-Röder A (2001) Synthesis 2001:171–196
Alexakis A, Benhaim C (2002) Eur J Org Chem 2002:3221–3236
Christoffers J, Baro A (2003) Angew Chem Int Ed 42:1688–1690
Jacobsen EN, Pfaltz A, Yamamoto H (1999) Comprehensive asymmetric catalysis. Springer, Berlin
Yamaguchi M, Shiraishi T, Hirama M (1993) Angew Chem Int Ed 32:1176–1178
Yamaguchi M, Shiraishi T, Hirama M (1996) J Org Chem 61:3520–3530
Halland N, Aburel PS, Jørgensen KA (2003) Angew Chem Int Ed 42:661–665
Wang J, Li H, Zu L, Jiang W, Xie H, Duan W, Wang W (2006) J Am Chem Soc 128:12652–12653
Dudziński K, Pakulska AM, Kwiatkowski P (2012) Org Lett 14:4222–4225
Wascholowski V, Knudsen KR, Mitchell CET, Ley SV (2008) Chem Eur J 14:6155–6165
Sasai H, Arai T, Satow Y, Houk KN, Shibasaki M (1995) J Am Chem Soc 117:6194–6198
Kim YS, Matsunaga S, Das J, Sekine A, Ohshima T, Shibasaki M (2000) J Am Chem Soc 122:6506–6507
Agostinho M, Kobayashi S (2008) J Am Chem Soc 130:2430–2431
Kim DY, Huh SC, Kim SM (2001) Tetrahedron Lett 42:6299–6301
Dere RT, Pal RR, Patil PS, Salunkhe MM (2003) Tetrahedron Lett 44:5351–5353
Ooi T, Ohara D, Fukumoto K, Maruoka K (2005) Org Lett 7:3195–3197
Wang Z, Wang Q, Zhang Y, Bao W (2005) Tetrahedron Lett 46:4657–4660
Li C, Feng X-W, Wang N, Zhou Y-J, Yu X-Q (2008) Green Chem 10:616–618
Li H-H, He Y-H, Yuan Y, Zhi G (2011) Green Chem 13:185–189
Brieva R, Crich JZ, Sih CJ (1993) J Org Chem 58:1068–1075
Xue Y, Li L-P, He Y-H, Guan Z (2012) Sci Rep 2:761. doi:10.1038/srep00761
Purkarthofer T, Gruber K, Gruber-Khadjawi M, Waich K, Skranc W, Mink D, Griengl H (2006) Angew Chem Int Ed 45:3454–3456
Sarma K, Goswami A, Goswami BC (2009) Tetrahedron 20:1295–1300
Faber K (2011) Biotransformations in organic chemistry: a textbook. Springer Science & Business Media, Berlin
Kourist R (2015) Biocatalysis in organic synthesis. Science of synthesis. Angew Chem Int Ed 54:12547
Humble MS, Berglund P (2011) Eur J Org Chem 2011:3391–3401
Wu Q, Liu B-K, Lin X-F (2010) Curr Org Chem 14:1966–1988
He T, Li K, Wu M-Y, Feng X-W, Wang N, Wang H-Y, Li C, Yu X-Q (2010) J Mol Catal B 67:189–194
Guan Z, Li L-Y, He Y-H (2015) RSC Adv 5:16801–16814
Müller M (2012) Adv Synth Catal 354:3161–3174
Busto E, Gotor-Fernández V, Gotor V (2010) Chem Soc Rev 39:4504–4523
Guan Z, Song J, Xue Y, Yang D-C, He Y-H (2015) J Mol Catal B 111:16–20
Yang F, Wang Z, Wang H, Zhang H, Yue H, Wang L (2014) RSC Adv 4:25633–25636
Liang Y-R, Chen X-Y, Wu Q, Lin X-F (2015) Tetrahedron 71:616–621
Wang J-L, Chen X-Y, Wu Q, Lin X-F (2014) Adv Synth Catal 356:999–1005
Cai Y, Sun X-F, Wang N, Lin X-F (2004) Synthesis 5:671–674
Cai Y, Wu Q, Xiao Y-M, Lv D-S, Lin XF (2006) J Biotechnol 121:330–337
Yao S-P, Lu D-S, Wu Q, Cai Y, Xu S-H, Lin XF (2004) Chem Commun 17:2006–2007
Priego J, Ortíz-Nava C, Carrillo-Morales M, López-Munguía A, Escalante J, Castillo E (2009) Tetrahedron 65:536–539
Carlqvist P, Svedendahl M, Branneby C, Hult K, Brinck T, Berglund P (2005) ChemBioChem 6:331–336
Svedendahl M, Hult K, Berglund P (2005) J Am Chem Soc 127:17988–17989
Strohmeier GA, Steinkellner G, Hartner FS, Andryushkova A, Purkarthofer T, Glieder A, Gruber K, Griengl H (2009) Tetrahedron 65:5663–5668
Xu J-M, Zhang F, Wu Q, Zhang Q-Y, Lin X-F (2007) J Mol Catal B 49:50–54
Xu K-L, Guan Z, He Y-H (2011) J Mol Catal B 71:108–112
Kitaume T, Ikeya T, Murata K (1986) J Chem Soc. Chem Commun 17:1331–1333
Kitazume T, Murata K (1988) J Fluorine Chem 39:75–86
Cai J-F, Guan Z, He Y-H (2011) J Mol Catal B 68:240–244
López-Iglesias M, Gotor-Fernández V (2015) Chem Rec 15:743–759
Wescott CR, Klibanov AM (1994) Biochim Biophys Acta 1206:1–9
Habulin M, Sabeder S, Paljevac M, Primozic M, Knez Z (2007) J Supercrit Fluids 43:199–203
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21472152 and 21672174), and the Basic and Frontier Research Project of Chongqing (cstc2015jcyjBX0106).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, LL., Li, LP., Xiang, Y. et al. Enzyme-Promoted Direct Asymmetric Michael Reaction by Using Protease from Streptomyces griseus . Catal Lett 147, 2209–2214 (2017). https://doi.org/10.1007/s10562-017-2095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2095-4