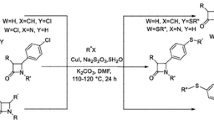
Abstract
A simple and convenient method has been developed for the construction of 3-thioindoles via molecular iodine-catalyzed direct thiolation of indoles with thiols. The present protocol, which employs thiols as the thiolating agents, inexpensive molecular iodine as the catalyst, and environmentally benign air as the oxidant, allows the regioselective generation of 3-thioindoles in good to excellent yields.
Graphical Abstract




Similar content being viewed by others
References
Katritzky AR, Pozharskii AF (2000) Handbook of heterocyclic chemistry. Pergamon Press, Oxford
Seigler DS (2001) Plant secondary metabolism. Springer, New York, p 628
Hibino S, Choshi T (2002) Nat Prod Rep 19:148
Takayama H, Tsutsumi SI, Kitajima M, Santiarworn D, Liawruangrath B, Aimi N (2003) Chem Pharm Bull 51:232
Humphrey GR, Tsutumi JT (2006) Chem Rev 106:2875
Campbell JA, Bordunov V, Borka CA, Browner MF, Kress JM, Mirzadegan T, Ramesha C, Sanpablo BF, Stabler R, Takahara P, Villasenor A, Walker KAM, Wang J-H, Welch M, Weller P (2004) Bioorg Med Chem Lett 14:4741
Holenz J, Pauwels PJ, Diaz JL, Mercè R, Codony X, Buschmann H (2006) Drug Discov Today 11:283
Kochanowska-Karamyan AJ, Hamann MT (2010) Chem Rev 110:4489
La Regina G, Edler MC, Brancale A, Kandil S, Coluccia A, Piscitelli F, Hamel E, De Martino G, Matesanz R, Díaz JF, Scovassi AI, Prosperi E, Lavecchia A, Novellino E, Artico M, Silvestri R (2007) J Med Chem 50:2865
Ragno R, Coluccia A, La Regina G, De Martino G, Piscitelli F, Lavecchia A, Novellino E, Bergamini A, Ciaprini C, Sinistro A, Maga G, Crespan E, Artico M, Silvestri R (2006) J Med Chem 49:3172
Khandekar SS, Gentry RD, Van Aller GS, Doyle ML, Chambers PA, Konstantinidis AK, Brandt M, Daines RA, Lonsdale JT (2001) J Biol Chem 276:30024
Berger JP, Doebber TW, Leibowitz M, Moller DE, Mostley RT, Tolman RL, Ventre J, Zhang BB, Zhou G (2001) PCT Int. Appl, WO 0130343
Acton JL, Meinke PT, Wood H, Black RM (2004) PCT Int Appl WO2004/019869 A2
Maeda Y, Koyabu M, Nishimura T, Uemura S (2004) J Org Chem 69:7688
De Martino G, Edler MC, La Regina G, Coluccia A, Barbera MC, Barrow D, Nicholson RI, Chiosis G, Brancale A, Hamel E, Artico M, Silvestri R (2006) J Med Chem 49:947
Cianchi F, Cortesini C, Magnelli L, Fanti E, Papucci L, Schiavone N, Messerini L, Vannacci A, Capaccioli S, Perna F, Lulli M, Fabbroni V, Perigli G, Bechi P, Masini E (2006) Mol Cancer Ther 5:2716
Luker T, Bonnert R, Brough S, Cook AR, Dickinson MR, Dougall I, Logan C, Mohammed RT, Paine S, Sanganee HJ, Sargent C, Schmidt JA, Teague S, Thom S (2011) Bioorg Med Chem Lett 21:6288
De Martino G, La Regina G, Collucia A, Edler MC, Barbera MC, Brancale A, Wilcox E, Hamel E, Artico M, Silvestri R (2004) J Med Chem 47:6120
Raban M, Chern L-J (1980) J Org Chem 45:1688
Hamel P, Préville P (1996) J Org Chem 61:1573
Wu Q, Zhao D, Qin X, Lan J, You J (2011) Chem Commun 47:9188
Chen M, Huang Z, Zheng Q (2012) Chem. Commun 48:11686
Kumaraswamy G, Raju R, Narayanarao V (2015) RSC Adv 5:22718
Yang F, Tian S (2013) Angew Chem Int Ed 52:4929
Li X, Xu Y, Wu W, Jiang C, Qi C, Jiang H (2014) Chem Eur J 20:7911
Browder CC, Mitchell MO, Smith RL, El-Stdayman G (1993) Tetrahedron Lett 34:6245
Li Z, Hong J, Zhou X (2011) Tetrahedron 67:3690
Silveira CC, Mendes SR, Wolf L, Martins GM, Mühlen L (2012) Tetrahedron 68:10464
Ge W, Wei Y (2012) Synthesis 44:934
Ge W, Wei Y (2012) Green Chem 14:2066
Huang D, Chen J, Dan W, Ding J, Liu M, Wu H (2012) Adv Synth Catal 354:2123
Sang P, Chen Z, Zou J, Zhang Y (2013) Green Chem 15:2096
Zhou X, Li X (2014) RSC Adv 4:1241
Tudge M, Tamiya M, Savarin C, Humphrey GR (2006) Org Lett 8:565
Silveira CC, Mendes SR, Wolf L, Martins GM (2010) Tetrahedron Lett 1:2014
Marcantoni E, Cipolletti R, Marsili L, Menichetti S, Properzi R, Viglianisi C (2013) Eur J Org Chem 1:132
Hostier T, Ferey V, Ricci G, Pardo DG, Cossy J (2015) Chem Commun 51:13898
Matsugi M, Gotanda K, Murata K, Kita Y (1997) Chem Commun 15:1387
Matsugi M, Murata K, Nambu H, Kita Y (2001) Tetrahedron Lett 42:1077
Xiao F, Xie H, Liu S, Deng G-J (2014) Adv Synth Catal 356:364
Rao H, Wang P, Wang J, Li Z, Sun X, Cao S (2014) RSC Adv 4:49165
Liu C, Ding L (2015) Org Biomol Chem 13:2251
Qi H, Zhang T, Wan K, Luo M (2016) J Org Chem 81:4262
Maeda Y, Koyabu M, Nishimura T, Uemura S (2004) J Org Chem 69:7688
Schlosser KM, Krasutsky AP, Hamilton HW, Reed JE, Sexton K (2004) Org Lett 6:819
Barraja P, Diana P, Carbone A, Cirrincione G (2008) Tetrahedron 64:11625
Wu G, Wu J, Wu J, Wu L (2008) Synth Commun 38:1036
Zhang X, Zhou X, Xiao H, Li X (2013) RSC Adv 3:22280
Liu Y, Zhang Y, Hu C, Wan J-P, Wen C (2014) RSC Adv 4:35528
Zhang H, Bao X, Song Y, Qu J, Wang B (2015) Tetrahedron 71:8885
Cui H, Liu X, Wei W, Yang D, He C, Zhang T, Wang H (2016) J Org Chem 81:2252
Cui H, Wei W, Yang D, Zhang J, Xu Z, Wen J, Wang H (2015) RSC Adv 5:84657
Wei W, Wen J, Yang D, Guo M, Wang Y, You J, Wang H (2015) Chem Commun 51:768
Wei W, Wen J, Yang D, Jing H, You J, Wang H (2015) RSC Adv 5:4416
Wei W, Liu X, Yang D, Dong R, Cui Y, Yuan F, Wang H (2015) Tetrahedron Lett 56:1808
Wei W, Wen J, Yang D, Du J, You J, Wang H (2014) Green Chem 16:2988
Wei W, Li J, Yang D, Wen J, Jiao Y, You J, Wang H (2014) Org Biomol Chem 12:1861
Wei W, Wen J, Yang D, Wu M, You J, Wang H (2014) Org Biomol Chem 12:7678
Wei W, Liu C, Yang D, Wen J, You J, Suo Y, Wang H (2013) Chem Commun 49:10239
Liu X, Cui H, Yang D, Dai S, Zhang T, Sun J, Wei W, Wang H (2016) RSC Adv 6:51830
Wen J, Wei W, Xue S, Yang D, Lou Y, Gao C, Wang H (2015) J Org Chem 80:4966
Hamdouchi C, Sanchez C, Ezquerra J (1998) Synthesis 6:867
Ravi C, Mohan DC, Adimurthy S (2014) Org Lett 16:2978
Mitra S, Ghosh M, Mishra S, Hajra A (2015) J Org Chem 80:8275
Bagdi AK, Mitra S, Ghosh M, Hajra A (2015) Org Biomol Chem 13:3314
Fang X-L, Tang R-Y, Zhong P, Li J-H (2009) Synthesis 24:4183
Liao Y, Jiang P, Chen S, Qi H, Deng G-J (2013) Green Chem 15:3302
Ge W, Zhu X, Wei Y (2013) Adv Synth Catal 355:3014
Azeredo JB, Godoi M, Martins GM, Silveira CC, Braga AL (2014) J Org Chem 79:4125
Vieira AA, Azeredo JB, Godoi M, Santi C, da Silva EN Jr, Braga AL (2015) J Org Chem 80:2120
Parumala SKR, Peddinti RK (2015) Green Chem 17:4068
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21302109, 21302110, and 21375075), the Taishan Scholar Foundation of Shandong Province, the Natural Science Foundation of Shandong Province (ZR2015JL004).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X., Cui, H., Yang, D. et al. Iodine-catalyzed Direct Thiolation of Indoles with Thiols Leading to 3-Thioindoles Using Air as the Oxidant. Catal Lett 146, 1743–1748 (2016). https://doi.org/10.1007/s10562-016-1798-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1798-2