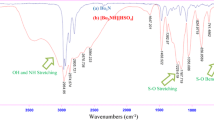
Abstract
This report has described three environmentally acceptable Brønsted acidic catalytic systems (p-toluene sulfonic acid, sulfamic acid and 1,3-disulfoimidazolium trifluoroacetate [DSIM][CF3COO] ionic liquid) for the preparation of complex library of anti-diastereomer of 2,3-dihydro-1,2,3-trisubstituted-1H-naphth [1,2-e][1,3]oxazines 4 from the three components reaction of 2-naphthol, p-substituted aromatic aldehydes and primary amines in 25 % aqueous ethanol at room temperature or in neat conditions at 80 °C. The reactions utilized 15 mol% of the two conventional acids to produce 82–97 % of naphthoxazines during 10–30 min. The same reactions could also be performed satisfactorily within 8–20 min employing 10 mol% of task specific acidic 1,3-disulfoimidazolium trifluoroacetate [DSIM][CF3COO] as catalyst. The three catalysts were recycled for four runs in aqueous ethanol with excellent catalytic activity.
Graphical Abstract



Similar content being viewed by others
References
Reddy DN, Prabhakaran EN (2011) J Org Chem 76:680
Turgut Z, Pelit E, Koyeu A (2007) Molecules 12:345
Butler JD, Solano DM, Robins LR, Haddadin MJ, Kurth MJ (2008) J Org Chem 73:234
Benameur L, Bouaziz Z, Nebois P, Bartoli MH, Boitard M, Fillion H (1996) Chem Pharm Bull 44:605
Mueller R, Li YX, Hampson A, Zhong S, Harris C, Marrs C, Rachwal R, Ulas J, Nielsson L, Rogers G (2011) Bioorg Med Chem Lett 21:3923
Cocuzza AJ, Chidester DR, Cordova BC (2011) Bioorg Med Chem Lett 11:1177
Mahato S, Haldar S, Jana CK (2014) Chem Commun 50:332
Larksarp C, Alper H (1999) J Org Chem 64:4152
Millan MJ, Di Cara B, Hill M, Jackson M, Joyce JN, Brotchie J, McGuire S, Crossman A, Smith L, Jenner P, Gobert A, Peglion JL, Brocco M (2004) J Pharmcol Exp Ther 309:921
Szatmari I, Martinek TA, Lazar L, Fulop F (2003) Tetrahedron 59:2877
Adib M, Sheibani E, Mostofi M, Ghanbary K, Bijanzadeh HR (2006) Tetrahedron 62:3435
Anzalone AV, Wang TY, Chen Z, Cornish VW (2013) Angew Chem Int Ed 52:650
Ruijterm E, Scheffelaar R, Orru RVA (2011) Angew Chem Int Ed 50:6234
Ganem B (2008) Acc Chem Res 42:463
Szatmari I, Fulop F (2004) Curr Org Synth 1:155
Cardellicchio C, Capozzi MAM, Naso F (2010) Tetrahedron Asymmetry 21:507
Szatmari I, Fulop F (2013) Tetrahedron 69:1255
Shinde PV, Kategaonkar AH, Shingate BB, Shingare MS (2011) Chin Chem Lett 22:915
Burke WJ, Nasutavicus WA, Weatherbee C (1962) J Org Chem 29:407
Sapkal SB, Shelke KF, Kategaonkar AH, Shingare MS (2009) Green Chem Lett Rev 2:57
Szatmari I, Martinek TA, Lazar L, Fulop F (2004) Eur J Org Chem 10:2231
Hanumanthu P, Ratnam CV (1977) Indian J Chem 15B:1019
Prabhakar V, Hanumanthu P, Ratnam CV (1982) Indian J Chem 21B:148
Li YH, Zhao MM, Zhang Y (2008) Acta Crystallogr Sect E 64:01972
Cimarelli C, Mazzanti A, Palmieri G, Volpini E (2001) J Org Chem 66:4759
Borah R, Dutta AK, Sarma P, Dutta C, Sarma B (2014) RSC Adv 4:10912
Horva’th I T, Anastas P T(2007) Chem Rev 107: 2169
Dallinger D, Kappe CO (2007) Chem Rev 107:2563
Martins MAP, Frizzo CP, Moreira DN, Zanatta N, Bonacorso HG (2008) Chem Rev 108:2015
Zhao G, Jiang T, Gao H, Han B, Huang J, Sun D (2004) Green Chem 6:75
Dutta AK, Gogoi P, Borah R (2014) RSC Adv 4:41287
Acknowledgments
The authors are thankful to Sophisticated Analytical Instrumentation Centre, Tezpur University, for analysis of various samples for this work and also to CSIR, New Delhi, India for granting a research project no. 02(0067)/12/EMR-II to the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10562_2015_1678_MOESM1_ESM.doc
This document includes the spectra of new product 4 and melting point of other known trisubstituted naphthoxazine derivatives. Supplementary material 1 (DOC 7899 kb)
Rights and permissions
About this article
Cite this article
Dutta, A.K., Gogoi, P., Saikia, M.P. et al. Development of Environmentally Benign Methods Towards the Synthesis of anti-2,3-dihydro-1,2,3-trisubstituted-1H-naphth[1,2-e][1,3]oxazines Using Brønsted Acidic Catalysts. Catal Lett 146, 902–908 (2016). https://doi.org/10.1007/s10562-015-1678-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1678-1