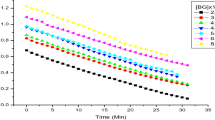
Abstract
Acid orange 7, chemically known as sodium 4-[(2E)-2-(2-oxonaphthalen-1-ylidene)hydrazinyl]benzenesulfonate, is extensively used for dyeing textiles, paper and leather. The discharge of wastewater containing this dye, causes environmental and health related problems. Therefore, in the present research, we have developed optimum conditions for the facile oxidative decolorization of this dye with sodium N-chlorobenzenesulfonamide or chloramine-B (CAB). The kinetics and mechanism of oxidative decolorization of acid orange 7 dye with CAB in acidic medium have also been studied spectrophotometrically at 303 K in the presence and absence of RuCl3 catalyst. Under similar experimental conditions, the reaction exhibits a first-order dependence of rate each on [CAB]o and [dye]o, and an inverse-fractional-order dependence on [H+] for both the RuCl3 catalyzed and uncatalyzed reactions. The order with respect to RuCl3 is fractional. Activation parameters have been computed. Dielectric effect is negative in both the cases. Oxidation products of the acid orange 7 dye are identified as 1,2-naphthoquinone and benzenesulfonic acid by GC–MS data. The RuCl3 catalyzed reaction is about four fold faster than the uncatalyzed reaction. The chemical oxygen demand value of the dye was determined. The mechanistic pathways and kinetic modelings have been computed based on experimental results. The developed oxidative decolorization method is expected to be helpful to treat acid orange 7 dye present in wastewater after suitable modifications.
Graphical Abstract
The stoichiometry of the reaction is in the mole ratio of 1:1(AO7:CAB) in both the cases as shown given below










Similar content being viewed by others
References
Zollinger H (1981) Color chemistry: synthesis, properties and applications of organic dyes and pigments. VCH, New York
Silva JP, Sousa S, Rodrigues J, Antunes H, Porter JJ, Goncalves I, Ferreira-Dias S (2004) Sep Purify Technol 40:309–315 and references therein
Li G, Wang N, Liu B, Zhang X (2009) Desalination 249:936–941
Yang S, Yang X, Shao X, Niu R, Wang L (2011) J Hazard Mater 186(1):659–666
Chen X, Qiao X, Wang D, Lin J, Chen J (2007) Chemosphere 67(4):802–808
Campbell MM, Johnson G (1978) Chem Rev 78:65–79
Banerji KK, Jayaram B, Mahadevappa DS (1987) J Sci Ind Res 46:65–76
Armesto XL, Canle L, Garia MV, Santaballa JA (1998) Chem Soc Rev 27:453–460
Agnihotri G (2005) Synlett 18:2857–2858
Kolaveri E, Ghorbeni-Choghamarani A, Salehi P, Shirini F, Zolfigol MA (2007) J Iran Chem Soc 4:126–174
Vinod KN, Puttaswamy, Gowda KNN (2009) Inorg Chim Acta 362:2044–2051
Puttaswamy, Sukhdev A, Shubha JP (2012) Prog React Kinet Mech 37:42–58
Puttaswamy, Jagadeesh RV (2006) Int J Chem Kinet 38:48–56
Puttaswamy, Jagadeesh RV (2005) Eur J Chem 3:482–501
Puttaswamy, Shubha JP (2008) Prog React Kinet Mech 33:313–330
Puttaswamy, Sukhdev A (2009) Indian J Chem 48:339–345
Griffith WP (1967) The chemistry of rare platinum metals. Interscience, New York
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York
Mallesh RT, Bellakki MB, Nandibewoor ST (2004) Catal Lett 97:91–98
Bhat KR, Jyothi K, Gowda BT (2002) Oxid Commun 25:117–141 and references therein
Rashmi R, Sushma G, Upadyay SK (1990) Indian J Chem 29A:847–851
Mulla RM, Hiremath GC, Nandibewoor ST (2004) Monatshefte fur Chemie 135:1489–1502
Jagadeesh RV, Puttaswamy (2008) J Phys Org Chem 21:844–858
Morris JC, Salazar JA, Wineman MA (1948) J Am Chem Soc 70:2036–2041
Puttaswamy, Shubha JP, Jagadeesh RV (2007) Trans Met Chem 32:991–999
Venkatesha BM, Ananda S, Mahadevappa DS (1992) J Phys Org Chem 5:373–381
Akerloff G (1932) J Chem Soc 54:4125–4139
Bishop E, Jennings VJ (1958) Talanta 1:197–199
Hardy FF, Johnston JP (1973) J Chem Soc Perkin Trans 2:742–745
Murthy ARV, Rao BS (1952) Proc Ind Acad Sci 35:69–72
Oakes J, Gratton P (1998) J Chem Soc Perkin Trans 2:2201 and reference therein
Cady HH, Connick RE (1958) J Am Chem Soc 80:2646–2652
Connick RE, Fine DA (1960) J Am Chem Soc 82:4187–4192
Backhouse JR, Dwyer FD, Shales N (1950) Proc R Soc 83:146–155
Davfokratova T (1963) Analytical chemistry of ruthenium. Academy of Sciences, USSR
Narayanan SS, Rao VRS (1983) Radiochem Acta 32:211–212
Subhashini M, Subramanian M, Rao VRS (1985) Talanta 32:1082–1085
Amis ES, Jaffe G (1942) J Chem Phys 10:598–604
Laidler KJ, Landskroener PA (1956) Trans Faraday Soc 52:200–210
Tanford C, Kirkwood JG (1957) J Am Chem Soc 79:5333–5339
Reihardt C (2003) Solvent and solvent effects in organic chemistry, 3rd edn. Wiley, New York
Laidler KJ (1995) Chemical kinetics, 2nd edn. Tata Mc-Graw Hill, New Delhi
Moelwyn-Hughes EA (1947) Kinetics of reactions in solutions. Oxford University Press, London
Gomati Devi L, Mohan Reddy K (2010) Appl Surf Sci 256:3116–3122
Acknowledgments
The authors greatly acknowledge the University Grant Commission, New Delhi for the award of UGC-Major Research Project [F. No. 39-721/2010 (SR)]. We thank Prof. M. A. Pasha of this department for his valuable suggestions regarding the reaction schemes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjunatha, A.S., Puttaswamy RuCl3 Catalyzed and Uncatalyzed Oxidative Decolorization of Acid Orange 7 Dye with Chloramine-B in Acid Medium: Spectrophotometric, Kinetic and Mechanistic Study. Catal Lett 145, 1312–1321 (2015). https://doi.org/10.1007/s10562-015-1526-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1526-3