Abstract
The oxidative kinetic resolution of racemic secondary alcohols was efficiently catalyzed by a chiral Mn(III)–salen complex using sodium hypochlorite (NaClO) as an oxidant in the presence of 8 mol% N-bromosuccinimide (NBS) in a dichloromethylene-water mixture solvent at room temperature. Excellent ee’s (up to 99 %) of chiral secondary alcohols were achieved in most cases.
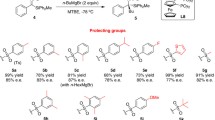
Graphical Abstract

Chiral Mn(III)–salen complex catalyzed oxidative kinetic resolution (OKR) of secondary alcohols has been achieved with sodium hypochlorite as an oxidant in the presence of a catalytic amount of N-bromosuccinimide. This novel protocol is very efficient for the OKR of a variety of secondary alcohols.



Similar content being viewed by others
References
Wills M (2008) Angew Chem Int Ed 47:4264
Laumen K, Breitgoff D, Schneider MP (1988) J Chem Soc Chem Commun 22:1459
Tang W, Zhang X (2003) Chem Rev 103:3029
Ikariya T, Blacker A (2007) J Acc Chem Res 40:1300
Morris RH (2009) Chem Soc Rev 38:2282
Ohkuma T (2010) Proc Jpn Acad Ser B 86:202
Theil F (1995) Chem Rev 95:2203
Breuer M, Ditrich K, Habicher T, Hauer B, Keßeler M, Stürmer R, Zelinski T (2004) Angew Chem Int Ed 43:788
Lee JH, Han K, Kim MJ, Park J (2010) Eur J Org Chem 6:999
Vedejs E, Jure M (2005) Angew Chem Int Ed 44:3974
Pellissier H (2011) Adv Synth Catal 35:1613
Zhang YC, Zhao SS, Mi GR, Zhao JQ (2012) Prog Chem 24:212
Xu D, Wang S, Shen Z, Xia CG, Sun W (2012) Org Biomol Chem 10:2730
Tan R, Dong Y, Peng M, Zheng WG, Yin DH (2013) Appl Catal A 458:1
Bera PK, Maity NC, Abdi SHR, Khan NH, Kureshy RI, Bajaj HC (2013) Appl Catal A 467:542
Ferreira EM, Stoltz BM (2001) J Am Chem Soc 123:7725
Jensen DR, Pugsley JS, Sigman MS (2001) J Am Chem Soc 123:7475
Chen T, Jiang JJ, Xu Q, Shi M (2007) Org Lett 9:865
Breuning M, Steiner M, Mehler C, Paasche A, Hein D (2009) J Org Chem 74:1407
Ebner DC, Trend RM, Matthew CG, McGrath J, O’Brien P, Stoltz BM (2008) Angew Chem Int Ed 47:6367
Ebner DC, Bagdanoff JT, Ferreira EM, McFadden RM, Caspi DD, Trend RM, Stoltz BM (2009) Chem Eur J 15:12978
Liu SJ, Liu LJ, Shi M (2009) Appl Organomet Chem 23:183
Nishibayashi Y, Yamauchi A, Onodera G, Uemura S (2003) J Org Chem 68:5875
Nakamura Y, Egami H, Matsumoto K, Uchida T, Katsuki T (2007) Tetrahedron 63:6383
Tanaka H, Nishikawa H, Uchida T, Katsuki T (2010) J Am Chem Soc 132:12034
Li YY, Zhang XQ, Dong ZR, Shen WY, Chen G, Gao JX (2006) Org Lett 8:5565
Arita S, Koike T, Kayaki Y, Ikariya T (2008) Angew Chem 120:2481
Arita S, Koike T, Kayaki Y, Ikariya T (2008) Angew Chem Int Ed 47:2447
Yamada T, Higano S, Yano T, Yamashita Y (2009) Chem Lett 38:40
Kunisu T, Oguma T, Katsuki T (2011) J Am Chem Soc 133:12937
Hamada T, Irie R, Mihara J, Hamachi K, Katsuki T (1998) Tetrahedron 54:10017
Sun W, Wang H, Xia CG, Li J, Zhao P (2003) Angew Chem Int Ed 42:1042
Li Z, Tang ZH, Hu XX, Xia CG (2005) Chem Eur J 11:1210
Sun W, Wu X, Xia CG (2007) HeIv Chim Acta 90:623
Cheng Q, Deng F, Xia CG, Sun W (2008) Tetrahedron Asymmetry 19:2359
Pathak K, Ahmad I, Abdi SHR, Kureshy RI, Khan NH, Jasra RV (2007) J Mol Catal A Chem 274:120
Kureshy RI, Ahmed I, Pathak K, Khan NH, Abdi SHR, Prathap JK, Jasra RV (2007) Chirality 19:352
Sahoo S, Kumar P, Lefebvre F, Halligudi SB (2008) Tetrahedron Lett 49:4865
Sahoo S, Kumar P, Lefebvre F, Halligudi SB (2009) Appl Catal A 354:17
Brown MK, Blewett MM, Colombe JR, Corey EJ (2010) J Am Chem Soc 132:11165
Zhang YC, Zhou Q, Ma WC, Zhao JQ (2014) Catal Commun 45:114
Larrow JF, Jacobsen EN (1994) J Org Chem 59:1939
Kantama ML, Ramania T, Chakrapani L, Choudary BM (2007) J Mol Catal A Chem 274:11
Han FR, Zhao JQ, Zhang YC, Wang WY, Zuo YY, An JW (2008) Carbohydr Res 343:1407
Krishnaveni NS, Surendra K, Rao KR (2004) Adv Synth Catal 346:346
Venkatasubramanian N, Thiagarajan V (1968) Tetrahedron Lett 14:1711
Venkatasubramanian N, Thiagarajan V (1967) Tetrahedron Lett 35:3349
Venkatasubramanian N, Thiagarajan V (1969) Can J Chem 47:694
Kruse PF, Grist KL, McCoy TA (1954) Anal Chem 26:1319
Acknowledgments
The authors are grateful for financial support from the National Natural Science Foundation of China (no. 21276061) and the Natural Science Foundation of Hebei Province, China (no. B2013202158).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Gao, B., Zhou, Q. et al. Oxidative Kinetic Resolution of Secondary Alcohols with Salen–Mn(III)/NBS/NaClO System. Catal Lett 144, 1797–1802 (2014). https://doi.org/10.1007/s10562-014-1339-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1339-9