Abstract
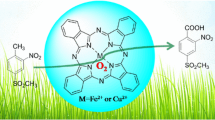
Iron(II) and palladium(II) phthalocyanines have been established as recyclable heterogeneous catalysts for the reduction of aromatic nitro compounds to corresponding amines using diphenylsilane/sodium borohydride as hydrogen sources in ethanol. Various reducible functional groups, such as acetyl, ester, cyano, amide, sulphonamide and carboxylic acid etc. were well tolerated, and the methods were applicable up to gram scale. Mechanistic studies showed that reduction of nitro group proceed through direct (nitroso) pathway and possibly iron or palladium phthalocyanines activates nitro group for reduction. FePc and PdPc also catalyzed the generation of hydrogen from the combination of diphenylsilane/sodium borohydride and ethanol.
Graphical Abstract
Iron and palladium (II) phthalocyanines has been established as an efficient recyclable catalytic systems for reduction of nitroarenes with green solvent system. Various nitro substituted aromatics and heteroaromatics has been successfully reduced to corresponding amines in good to excellent yields. The present methods have also been productively applicable for gram scale reactions.




Similar content being viewed by others
References
Rylander PN (1985) Hydrogenation Methods. Academic Press, London 104
Nishimura S (2001) Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis. Wiley, Chichester 315
Adams JP, Paterson JR (2000) J Chem Soc Perkin Trans 1:3695
Kabalka GW, Verma RS (1992) In: Comprehensive Organic Synthesis. Pergamon, Oxford 363
Dale DJ, Dunn PJ, Golighty C, Hughes ML, Levett PC, Pearce AK, Searle PM, Ward G, Wood AS (2000) Org Process Res Dev 4:17
Brickner SJ, Hutchinson DK, Barbachyn MR, Manninen PR, Ulanowicz DA, Garmon SA, Grega KC, Hendges SK, Toops DS, Ford CW, Zurenko GE (1996) J Med Chem 39:673
Al-Farhan E, Deininger DD, McGhie S, Callaghan JO, Robertson MS, Rodgers K, Rout SJ, Singh H, Tung RD (1999) PCT Int. Appl. WO99/48885
Prasad A, Sharma ML, Kanwar S, Rathee R, Sharma SD (2005) J Sci Ind Res 64:756
Blaser HU, Siegrist U, Studer M (2001) In: Fine chemicals through heterogenous catalysis. Wiley-VCH, Weinheim 389
Blaser HU, Steiner H, Studer M (2009) ChemCatChem 1:210
Corma A, Serna P, Concepcion P, Calvino J (2008) J Am Chem Soc 130:8748
Doxsee KM, Feigel M, Stewart KD, Canary JW, Knobler CB, Cram DJ (1987) J Am Chem Soc 109:3098
Tormo J, Hays DS, Fu GC (1998) J Org Chem 63:5296; c) Zhou Y, Li J, Liu H, Zhao Z, Jiang H (2006). Tetrahedron Lett 47:8511
Sharma U, Kumar P, Kumar N, Kumar V, Singh B (2010) Adv Synth Catal 352:1834
Sahiner N, Ozay H, Ozay O, Aktas N (2010) Appl Catal B Env 101:137
Matthews JM, Greco MN, Hecker LR, Hoekstra WJ, Rade-Gordon P, de Garavilla L, Demarest KT, Ericson K, Gunnet KW, Hageman W, Look R, Moore JB, Maryanoff BE (2003) Bioorg Med Chem Lett 13:753
Kim Y, Nam NH, You YJ, Ahn BZ (2002) Bioorg Med Chem Lett 12:719
Edwards JP, Zhi L, Pooley CLF, Tegley CM, West SJ, Wang MW, Gottardis MM, Pathirana C, Schrader WT, Jones TK (1998) J Med Chem 41:2779
Neidlein R, Christen D (1986) Helv Chim Acta 69:1623
Liu Y, Lu Y, Prashad M, Repic O, Blacklock TJ (2005) Adv Synth Catal 347:217
Corma A, Conceptcion P, Serna P (2007) Angew Chem Int Ed 46:7266
He L, Wang LC, Sun H, Ni J, Cao Y, He HY, Fan KN (2009) Angew Chem Int Ed 48:9538
Corma A, Serna P (2006) Science 313:332
Corma A, Serna P, Garcia H (2007) J Am Chem Soc 129:6358
Park S, Lee IS, Park J (2013) Org Biomol Chem 11:395
Mitsudome T, Kaneda K (2013) Green Chem 15:2636
Zhang Y, Cui X, Shi F, Deng Y (2012) Chem Rev 112:2467
Stratakis M, Garcia H (2012) Chem Rev 112:4469
Gkizis PL, Stratakis M, Lykakis IN (2013) Catal Commun 36:48
Lipowitz J, Bowman SA (1973) J Org Chem 38:162
Jovel I, Golomba L, Fleisher M, Popelis J, Grinberga S, Lukevics E (2004) Chem Heterocycl Comp 40:701
Rahaim Jr RJ, Maleczka Jr. RE (2006) Synthesis 3316
Rahaim RJ Jr, Maleczka RE Jr (2005) Org Lett 7:5087
Banik BK, Mukhopadhyay C, Venkatraman MS, Becker FF (1998) Tetrahedron Lett 39:7243
Yu C, Liu B, Hu L (2001) J Org Chem 66:919
Basu MK, Becker FF, Banik FF (2000) Tetrahedron Lett 41:5603
Spencer J, Anjum N, Patel H, Rathnam RP, Verma J (2007) Synlett 2557
Spencer J, Rathnam RP, Patel H, Anjum N (2008) Tetrahedron 64:10195
de Noronha RG, Romao CC, Fernandes AJ (2009) J Org Chem 74:6960
Andrianov KA, Sidorov VI, Filimonov MI (1977) Zh Obshch Khim 47:485
Brinkman HR, Miles WH, Hilborn MD, Smith MC (1996) Synth Commun 26:973
Fan GY, Zhang L, Fu HY, Yuan ML, Li RX, Chen H, Li XJ (2010) Catal Commun 11:451
Enthaler S, Junge K, Beller M (2008) Angew Chem Int Ed 47:3317
Gaillard S, Renaud JL (2008) ChemSusChem 1:505
Junge K, Wendt B, Shaikh N, Beller M (2010) Chem Commun 46:1769
Pehlivan L, Metay E, Laval S, Dayoub W, Demonchaux P, Mignani G, Lemaire M (2011) Tetrahedron 67:1971
Cantillo D, Baghbanzadeh M, Kappe CO (2012) Angew Chem Int Ed 51:10190
Wienhofer G, Sorribes I, Boddien A, Westerhaus F, Junge K, Junge H, Llusar R, Beller M (2011) J Am Chem Soc 133:12875
Shi Q, Lu R, Lu L, Fu X, Zhao D (2007) Adv Synth Catal 349:1877
Plietker B (2008) Iron catalysis in organic chemistry. Wiley-VCH, Weinheim
Nahra F, Mace Y, Lambin D, Riant O (2013) Angew Chem Int Ed 52:3208
Wang DS, Wang DW, Zhou YG (2011) Synlett 947
Bae JW, Cho YJ, Lee SH, Yoon COM, Yoon CM (2000) Chem Commun 1857
Franzoni I, Mazet C (2014) Org Biomol Chem 12:233
Chen QA, Ye ZS, Duan Y, Zhou YG (2013) Chem Soc Rev 42:497
Sorokin AB (2013) Chem Rev 13:8152
Verma PK, Sharma U, Bala M, Kumar N, Singh B (2013) RSC Adv 3:895
Sharma U, Kumar N, Verma PK, Kumar V, Singh B (2012) Green Chem 14:2289
Sharma U, Verma PK, Kumar N, Kumar V, Bala M, Singh B (2011) Chem Eur J 17:5903
Bala M, Verma PK, Kumar N, Sharma U, Singh B (2013) Canad J Chem 91:732
Bala M, Verma PK, Sharma U, Kumar N, Singh B (2013) Green Chem 15:1687
Verma PK, Sharma U, Kumar N, Bala M, Kumar V, Singh B (2012) Catal Lett 142:907
Kumar V, Sharma U, Verma PK, Kumar N, Singh B (2012) Adv Synth Catal 354:870
Kantam ML, Bandyopadhyay P, Rahman A (1998) J Mol Catal A: Chem 133:293
McLaughlin MA, Barnes DM (2006) Tetrahedron Lett 47:9095
Tafesh AM, Weiguny J (1996) Chem Rev 96:2035
Takasaki M, Motoyama Y, Higashi K, Yoon, Mochida I, Nagasimha H (2008) Org Lett 10:1601
Sorribes I, Wienhofer G, Vicent C, Junge K, Llusar R, Beller M (2012) Angew Chem Int Ed 51:7794
Westerhaus FA, Jagadeesh RV, Wienhofer G, Pohl MM, Radnik J, Surkus AE, Robeah J, Junge K, Junge H, Nielsen M, Bruckner A, Beller M (2013) Nature Chem 5:537
Lee JG, Choi KI, Koh HY, Kim Y, Kang Y, Cho YS (2001) Synthesis 81
Chandrasekhar S, Prakash SJ, Rao CL (2006) J Org Chem 71:2196
Iyer S, Kulkarni GM (2004) Synth Commun 34:721
He D, Shi H, Wu Y, Xu BO (2007) Green Chem 9:849
Weekes AA, Westwell AD (2009) Curr Med Chem 16:2430
Horton DA, Bourne GT, Smythe MY (2003) Chem Rev 103:893
Kuhler TC, Swanson M, Shcherbuchin V, Larsson H, Mellgard B, Sjostrom JE (1998) J Med Chem 41:1777
Haber F (1898) Z Elektrochem 22:506
Kruger A, Albrecht M (2012) Chem Eur J 18:652
Mukharjee D, Thompson RR, Ellern A, Sadow AD (2011) ACS Catal 1:698
Weickgenannt A, Mewald M, Muesmann TWT, Oestreich M (2010) Angew Chem Int Ed 49:2223
Ito H, Takagi K, Miyahara T, Sawamura M (2005) Org Lett 7:3001
Ito H, Takagi K, Miyahara T, Sawamura M (2005) Org Lett 7:1869
Khalimon AY, Simionescu R, Nikonov GI (2011) J Am Chem Soc 133:7033
Luo XL, Crabtree RH (1989) J Am Chem Soc 111:2527
Bialek B, Lee J (2007) J Korean Phys Soc 51:1366
Acknowledgments
Authors are grateful to Director of the institute for providing necessary facilities. Financial support received from CSIR-India (fellowship to P. K. V) and DST under Fast Track Scheme (U. S.) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
CSIR-IHBT Communication No. 3564.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, P.K., Bala, M., Thakur, K. et al. Iron and Palladium(II) Phthalocyanines as Recyclable Catalysts for Reduction of Nitroarenes. Catal Lett 144, 1258–1267 (2014). https://doi.org/10.1007/s10562-014-1269-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1269-6