Abstract
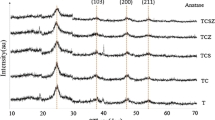
In the current study, it was found that the physiochemical properties of TiO2 are effected with formic acid as a chelating agent in a sol–gel process. From XRD studies it was revealed that due to the chelation of formate group with titanium precursor, anatase/brookite mixture of 86:14 is obtained while the control sample which has been prepared without formic acid showed pure anatase at 400 °C. FTIR studies indicated that, the formate group favored a monodentate mode of coordination with titanium precursor under the effect of addition of increasing amount of formic acid, while under the effect of increasing titanium precursor content the formate group is chelated with titanium atoms in a bidentate bridging mode. In addition, from the FTIR study it was demonstrated that increasing the amount of water in the hydrolysis step, a reduction in the intensity of the carboxylate (COO−) stretches was observed indicating that the titania formate bridging complex becomes weaker, resulting in a weakened titanium gel network structure which could readily collapse during calcination favoring early rutile formation. Raman spectroscopy showed that as a result of sample composite nature and presence of oxygen vacancies as demonstrated by PL analysis causes the broadening and frequency shifting of the Raman bands. Photocatalytic studies demonstrated that the formic acid modified sample composed of anatase/rutile mixture of 94:6 calcined at 600 °C show significantly higher catalytic activity compared to the control sample prepared under similar conditions. Kinetic analysis show first order kinetics for the decomposition of methylene blue, a rate constant (kapp) of 0.074 min−1 was obtained with anatase/rutile (94:6) mixture which is even higher than the Degussa P25 (0.067 min−1).
Graphical Abstract










Similar content being viewed by others
Abbreviations
- ART:
-
Anatase to rutile transformation temperature
- Δ:
-
Delta represent the difference in asymmetric (ν(COO−)as) and symmetric carboxylate (–ν(COO−)s)) stretches
- C:
-
Reaction concentration
- Co :
-
Concentration of organic pollutant
- Kapp :
-
Apparent reaction rate constant
- CB:
-
Conduction band
- VB:
-
Valance band
- FWHM:
-
Full width at half maximum
- α:
-
Absorption coefficient
- Eu :
-
Urbach energy
- hυ:
-
Photon energy
- λ:
-
Wavelength
References
Ying L, Hon LS, White T, Withers R, Hai LB (2003) Mater Trans 44:1328
Sahni S, Reddy SB, Murty BS (2007) Mater Sci Eng, A 452–453:758
Xu N, Shi Z, Fan Y, Dong J, Shi J, Hu MZC (1999) J Ind Eng Chem Res 38:373
Mao L, Li Q, Dang H, Zhang Z (2005) Mater Res Bull 40:201
Zhu J, Zhang J, Chen F, Iino K, Anpo M (2005) Top Catal 35:261
Zhang D, Qi L, Ma J, Cheng H (2002) J Mater Chem 12:3677
Schaefer DW, Martin JE, Wiltzius P, Cannell DS (1984) Aggregation of colloidal silica. In: Family F, Landau DP (eds) Kinetics of aggregation and gelation. Elsevier, Amsterdam
Yu Y, Xu D (2007) J Appl Catal B Environ 73:166
Nguyen TV, Choi DJ, Yang OB (2005) Res Chem Intermed 31:483
Suresh C, Biju V, Mukundan P, Warrier KGK (1998) Polyhedron 17:3131
Yin H, Wada Y, Kitamura T, Kambe S, Murasawa S, Mori H, Sakata T, Yanagida S (2001) J Mater Chem 11:1694
Zhang H, Banfield JF (2000) J Phys Chem B 104:3481
Chen Z, Zhao G, Li H, Han G, Song B (2009) J Am Ceram Soc 92:1024
Liu Y, Wang Z, Wang W, Huang W (2014) J. Cataly 310:16
Bischoff BL, Anderson MA (1995) Chem Mater 7:1772
Zelenak V, Vargova Z, Gyoryova K (2007) Spectrochim Acta Mol Biomol Spectros 66:262
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordinated compounds, part b: application in coordination, organometallic and bioinorganic chemistry. John Wiley and Sons Inc., Hoboken
Nolan NT, Seery MK, Pillai SC (2009) J Phys Chem C 113:16151
Coronado DR, Gattorno GR, Pesqueira MEE, Cab C, Coss R, Oskam G (2008) Nanotechnology 19:145605
Valencia S, Marin JM, Restrepo G (2010) Open Mater Sci J 4:9
Deng H, Hossenlopp JM (2004) J Phys Chem B 109:66
Mathpal MC, Tripathi AK, Singh MK, Gairola SP, Pandey SN, Agarwal A (2013) Chem Phys Lett 555:182
Serpone N, Lawless D, Khairutdinov R (1995) J Phys Chem 99:16646
Bouras P, Stathatos E, Lianos P (2007) Appl Catal B Environ 73:51
Wang Y, Zhang S, Wu X (2004) Nanotechnology 15:1162
Choudhury B, Dey M, Choudhury A (2013) Shallow and deep trap emission and luminescence quenching of TiO2 nanoparticles on Cu doping. Appl Nanosci. doi:10.1007/s13204-013-0226-9
Li D, Haneda H, Hishita S, Ohashi N, Labhsetwar N (2005) J Fluorine Chem 126:69
Khan H, Berk D (2013) J Sol-Gel Sci Technol 68:1
Xu J, Shi S, Li L, Zhang X, Wang Y, Chen X, Wang J, Lv L, Zhang F, Zhong W (2010) J Appl Phys 107:053910
Zhou J, Takeuchi M, Ray AK, Anpo M, Zhao XS (2007) J Colloid Interface Sci 311:497
Choi HC, Jung YC, Kim SB (2005) Vib Spectr 37:33
Yan J, Wu G, Guan N, Li L, Li Z, Cao X (2013) Phys Chem Chem Phys 15:10978
Jensen H, Pedersen JH, Jorgensen JE, Pedersen JS, Joensen KD, Iversen SB, Sogaard EG (2006) J Exp Nanosci 1:355
Yu J, Yu H, Cheng B, Zhou M, Zhao X (2006) J Mol Catal A: Chem 253:112
Ardizzone S, Bianchi CL, Cappelletti G, Gialanella S, Pirola C, Ragaini V (2007) J Phys Chem C 111:13222
Chen DC, Wang Z, Liao ZF, Maic YL, Zhang MQ (2007) Polym Test 26:202
Asenjo NG, Santamaria R, Blanco C, Granda M, Alvarez P, Menendez R (2013) Carbon 55:62
Qourzal S, Barka N, Tamimi T, Assabbane A, Ichou YA (2008) Appl Catal A Gen 334:386
Acknowledgments
Authors would like to acknowledge Natural Sciences and Engineering Research Council (NSERC) of Canada for the financial support provided for this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, H., Berk, D. Effect of a Chelating Agent on the Physicochemical Properties of TiO2: Characterization and Photocatalytic Activity. Catal Lett 144, 890–904 (2014). https://doi.org/10.1007/s10562-014-1233-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1233-5