Abstract
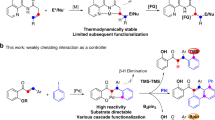
The phoszone ligand [(Ph2P)(bis-3,5-CF3-Ph)]NN=CH(penta-fluoro-Ph) transformed in liquid CO2 at room temperature in presence of [Rh(cod)2]OTf into [Rh(cod)(η2-P,P′-Ph2POPPh2)]OTf. Replacing the O-atom in Ph2POPPh2 by a PrN-group leads to the ligand PrN(PPh2)2 acting similarly as a bidentate ligand in [Rh(cod)(η2-P,P′-PrN(PPh2)2)]OTf. Hydroformylation of 1-octene with in situ catalysts formed by the ligands with [Rh(cod)2]OTf showed hydroformylation activities at 50 % conversion of 16,000 h−1 (PrN(PPh2)2/[Rh(cod)2]OTf) and 24,000 h−1 (phoszone/[Rh(cod)2]OTf), respectively.
Graphical Abstract











Similar content being viewed by others
Notes
Structural details for 2*OTf: Reflections collected/unique/observed (I > 2σ): 40,467/15,873/12,529 [R(int) = 0.222]; parameters refined: 800; formula C66H65F6O8P4Rh2S2 (two molecules), MM per molecule 747.0 g/mol; T = 200(2) K; triclinic, P-1 (No. 2, Z = 2); a = 1,149.5(1) pm; b = 1,515.5(1) pm; c = 2,049.6(2) pm; α = 71.784(1)°; β = 78.518(1)°; γ = 70.494(1)°; V = 3,179.2(5) × 106 pm3; ρ (calc.) = 1.561 g/cm3; Absorption coefficient = 0.758 mm−1; F(000) = 1,522; Goof (F2) = 1.035; crystal size = 0.8 × 0.8 × 0.6 mm3; index ranges −16 < h < 16, −21 < k < 20, −28 < l < 28; completeness to θ = 28.34: 95.2 %; R1 (I > 2σ) = 0.0316, wR2 = 0.0851 (all data), largest difference peak and hole: 0.836 and −0.513 × 10−6 pm−3. Structural details for 4*OTf*CDCl3: Reflections collected/unique/observed: 25,128/9,067/8,250 [R(int) = 0.0317]; parameters refined: 467; formula C37H40Cl3F3NO3P2RhS, MM 906.96 g/mol; T = 200(2) K; monoclinic, Pn (No. 7, Z = 2); a = 1,158.8(3) pm; b = 944.1(3) pm; c = 1,815.7(5) pm; β = 105.465(5)°; V = 1,914(1) × 106 pm3; ρ (calc.) = 1.573 g/cm3; Absorption coefficient = 0.846 mm−1; F(000) = 924; Goof (F2) = 1.014; crystal size = 0.6 × 0.6 × 0.5 mm3; index ranges −15 < h < 15, −12 < k < 12, −24 < l < 24; completeness to θ = 28.34: 97.7%; R1 (I > 2σ) = 0.0307 wR2 = 0.0698 (all data), largest difference peak and hole: 0.653 and −0.325 × 10−6 pm−3.
References
Franke R, Selent D, Börnera A (2012) Chem Rev 112:5675
Ungvary F (2007) Coord Chem Rev 251:2072
Van Rooy A, Orij EN, Kamer PCJ, Van Leeuwen PWNM (1995) Organometallics 14:34
Van Rooy A, de Bruijn JNH, Roobeek KF, Kamer PCJ, Van Leeuwen PWNM (1996) J Organomet Chem 507:69
Van Leeuwen PWNM, Roobeek CF (1983) J Organomet Chem 258:343
Jongsma T, Challa G, Van Leeuwan PWNM (1991) J Organomet Chem 421:121
Van Rooy A, Orij EN, Kamer PCJ, Van Den Aardweg F, Van Leeuwen PWNM (1991) J Chem Soc Chem Commun 16:1096
Desset SL, Cole-Hamilton DJ (2009) Angew Chem Int Ed 49:1472
van der Slot SC, Kamer PCJ, van Leeuwen PWNM, Fraanje J, Goubitz K, Lutz M, Spek AL (2000) Organometallics 19:2504
Magee MP, Luo W, Hersh WH (2002) Organometallics 21:362
E. Piras, Dissertation no. 16317 (ETH Zürich, 2005)
Dahmen N, Griesheimer P, Makarczyk P, Pitter S, Walter O (2006) J Organomet Chem 690:1467
Kainz S, Koch D, Baumann W, Leitner W (1997) Angew Chem Int Ed Engl 36:1628
Koch D, Leitner W (1998) J Am Chem Soc 120:13398
Wong EH, Prasad L, Gabe EJ, Bradley FC (1982) J Organomet Chem 236:321
Bradley FC, Wong EH, Gabe EJ, Lee FL, Lepage Y (1987) Polyhedron 6:1103
Pavlik S, Mereiter K, Puchberger M, Kirchner K (2005) Organometallics 24:3561
Irvine DJ, Cole-Hamilton DJ, Barnes J, Hodgson PKG (1989) Polyhedron 8:1575
Irvine DJ, Glidewell C, Cole-Hamilton DJ, Barnes JC, Howie A (1991) J Chem Soc Dalton Trans 263:1765
Bravo J, Castro J, Garcia-Fontan S, Rodriguez-Martinez MC, Rodriguez-Seoane P (2006) Eur J Inorg Chem 3028
Domide D, Kaifer E, Mautz J, Walter O, Behrens S, Himmel HJ (2008) Eur J Inorg Chem 3177
Hoffmann R (1981) Science 211:95
Durap F, Biricik N, Gumgum B, Ozkar S, Ang WH, Fei Z, Scopelliti R (2008) Polyhedron 27:96
Gallo V, Mastrorilli P, Nobile CF, Braunstein P, Englert U (2006) Dalton Trans 8:2342
Gumgum B, Biricik N, Durap F, Ozdemir I, Gurbuz N, Ang WH, Dyson P (2007) J Appl Organomet Chem 21:11
Bowen LE, Haddow MF, Orpen AG, Wass DF (2007) Dalton Trans 1160
Simon-Manso E, Valderrama M (2006) J Organomet Chem 691:380
Valderrama M, Contreras R, Boys D (2003) J Organomet Chem 665:7
Posset T, Rominger F, Blümel J (2005) Chem Mater 17:586
Ewart G, Lane AP, McKechnie J, Payne DS (1964) J Chem Soc 1543
Yip SK, Lam WH, Zhu N, Yam VWW (2006) Inorg Chim Acta 359:3639
Balakrishna MS, Teipel S, Pinkerton AA, Cavell RG (2001) Inorg Chem 40:1802
Walter O, Huttner G, Kern R (1996) Z Naturforsch B 51:922
C. Claver, P.W. van Leeuwen, Rhodium Catalyzed Hydroformylation (Springer; 2000)
Maura R, Steele J, Vendier L, Arquier D, Bastin S, Urrutigoïty M, Kalck P, Igau A (2011) J Organomet Chem 696:897
Ionescu C (2009) Katalyse und Katalysatoren zur Hydroformylierung langkettiger Olefine in überkritischem Kohlendioxid. Dissertation, Universität Heidelberg
Gorschinski A, Habicht W, Walter O, Behrens S, Ionescu C, Powietzka B (in preparation)
Maniut C (2007), Reaktionstechnische Aspekte zur Hydroformylierung in überkritischem Kohlendioxid. Dissertation, Universität Heidelberg
F. Wurst, Überführung der Hydroformylierung mit immobilisierten Rhodium-Katalysatoren in ein kontinuierliches Verfahren im Labormaßstab. (Dissertation, Universität Heidelberg, to be concluded in 2013)
Heck RF, Breslow DS (1961) J Am Chem Soc 83:4023
Evans D, Yagupsky G, Wilkinson G (1968) J Chem Soc A 3133
SADABS, Siemens area detector absorption correction programme (Siemens, 1997)
SHELX-97, G. Sheldrick (University of Göttingen, Germany, 1997)
Xpma L, Zsolnai G (1997) Huttner. University of Heidelberg, Germany
Winray R, Soltek G (1997) Huttner. University of Heidelberg, Germany
Acknowledgment
The authors would like to express their deepest thanks to the KIT and the ETH Zürich for the financial and Prof. Dr. Eckhard Dinjus for the general support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Prof. Ivano Bertini.
Rights and permissions
About this article
Cite this article
Piras, E., Powietzka, B., Wurst, F. et al. Highly Active Hydroformylation Catalysts: Synthesis, Characterisation and Catalytic Performance. Catal Lett 143, 673–680 (2013). https://doi.org/10.1007/s10562-013-1010-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1010-x