Abstract
We investigated the effect of loading potassium over iron-based catalysts for water–gas shift (WGS) reaction at 573 K. The iron-based water–gas shift catalyst has been known as a redox-mechanism catalyst. Therefore, to promote the redox reaction over iron oxide, we impregnated a small amount of Pd on various iron oxides. Results revealed that coexisting potassium and palladium accelerated the WGS reaction over iron-oxide catalyst. We examined the optimum amount of potassium over Pd/iron catalyst, and we found that the optimum molar ratio was about 2 (K/Pd molar ratio). From the viewpoint of reducibility of the catalyst, the addition of small amount of Pd onto iron oxide promotes reduction from Fe2O3 to Fe3O4. However, impregnation of potassium on iron oxide makes the catalyst structure robust. The promotion effect of Pd and potassium was not observed on SiO2 support. We therefore concluded that the synergetic effect among Pd, K, and iron oxide, was important for the WGS reaction.
Graphical abstract
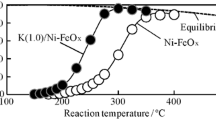
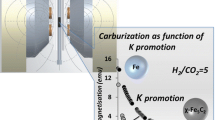
Water-gas shift reaction over iron-based catalyst at 573 K; the effect of loading amount of potassium over Pd/Fe2O3 catalysts.







Similar content being viewed by others
References
Bharadwaj SS, Schmidt LD (1995) Fuel Process Technol 42:109
Ghenciu AF (2002) Curr Opinion Solid State Mater Sci 6:389
Gorte RJ, Zhao S (2005) Catal Today 104:18
Liu X, Ruettinger W, Xu X, Frrauto R (2005) Appl Catal B 56:69
Wang X, Gorte RJ, Wagner JP (2002) J Catal 212:225
Andreeva D, Idakiev V, Tabakova T, Ilieva L, Falaras P, Bourlinos A, Travlos A (2002) Catal Today 72:51
Sekine Y, Takamatsu H, Aramaki S, Ichishima K, Takada M, Matsukata M, Kikuchi E (2009) Appl Catal A 352:214
Twigg MV, Spencer MS (2001) Appl Catal A 212:161
Zalc JM, Sokolovskii V, Löffler DG (2002) J Catal 206:169
Trimm DL (2005) Appl Catal A 296:1
Liu Q, Ma W, He R, Mu Z (2005) Catal Today 106:52
Ratnasamy C, Wagner JP (2009) Catal Rev Sci Eng 51:325
Idakiev V, Mihajlova D, Kunev B, Andreev A (1987) React Kinet Catal Lett 33:119
Lei Y, Cant NW, Trimm DL (2005) Catal Lett 103:133
Rhodes C, Williams BP, King F, Hutchings GJ (2002) Catal Commun 3:381
Natesakhawat S, Wang X, Zhang L, Ozkan US (2006) J Mol Catal A Chem 260:82
Zhang L, Wang X, Millet JMM, Matter PH, Ozkan US (2008) Appl Catal A 351:1
Watanabe R, Sekine Y, Aramaki S, Takamatsu H, Matsukata M, Kikuchi E (2010) Top Catal 53:621
Urasaki K, Tanimoto N, Hayashi T, Sekine Y, Kikuchi E, Matsukata M (2005) Appl Catal A 288:143
Rhodes C, Hutchings GJ, Ward AM (1995) Catal Today 23:43
Basinska A, Domka F (1999) React Kinet Catal Lett 67:111
Jozwiak WK, Maniecki TP, Basinska A, Goralski J, Fiedorow R (2004) Kinet Catal 45:879
Yang Y, Xiang HW, Xu YY, Bai L, Li YW (2004) Appl Catal A 266:181
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sekine, Y., Chihara, T., Watanabe, R. et al. Effect of Loading Potassium and Palladium over Iron-Based Catalyst for Low Temperature Water–Gas Shift Reaction. Catal Lett 140, 184–188 (2010). https://doi.org/10.1007/s10562-010-0444-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0444-7