Abstract
Methane steam reforming is the key reaction to produce synthesis gas and hydrogen at the industrial scale. Here the kinetics of methane steam reforming over a rhodium-based catalyst is investigated in the temperature range 500–800 °C and as a function of CH4, H2O and H2 partial pressures. The methane steam reforming reaction cannot be modeled without taking CO and H coverages into account. This is especially important at low temperatures and higher partial pressures of CO and H2. For methane CO2 reforming experiments, it is also necessary to consider the repulsive interaction of CO that lowers the adsorption energy at high CO coverage. The CO–CO interaction is supported by comparison with fundamental surface science studies.
Graphical Abstract
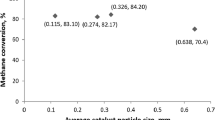
Experimental results (points), Langmuir–Hinshelwood kinetic modeling (lines) and descriptive power law constants for the methane dependency in the methane steam reforming reaction on a Rh catalyst.








Similar content being viewed by others
References
Rostrup-Nielsen JR (2000) Catal Today 63:159–164
Rostrup-Nielsen JR, Sehested J, Nørskov JK (2002) Adv Catal 47:65–139
Rostrup-Nielsen JR (1984) In: Anderson JR, Boudart M (eds) Catalysis—science and technology. Springer, Berlin
Rostrup-Nielsen JR, Hansen J-HB (1993) J Catal 144:38–49
Kikuchi E, Tanaka S, Yamazaki Y, Morita Y (1974) Bull Jpn Pet Inst 95–98
Qin D, Lapszewicz J (1994) Catal Today 21:551–560
Rostrup-Nielsen JR (1973) J Catal 31:173–199
Jones G, Jakobsen JG, Shim SS, Kleis J, Andersson MP, Rossmeisl J, Abild-Pedersen F, Bligaard T, Helveg S, Hinnemann B, Rostrup-Nielsen JR, Chorkendorff I, Sehested J, Nørskov JK (2008) J Catal 259:147–160
Wei JM, Iglesia E (2004) J Catal 224:370–383
Jakobsen JG, Jørgensen TL, Chorkendorff I, Sehested J (2010) Appl Catal A 377:158–166
Bhat RN, Sachtler WMH (1997) Appl Catal A 150:279–296
Wang HY, Ruckenstein E (2000) Appl Catal A 204:143–152
Hei MJ, Chen HB, Yi J, Lin YJ, Lin YZ, Wei G, Liao DW (1998) Surf Sci 417:82–96
Munera JF, Cornaglia LM, Cesar DV, Schmal M, Lombardo EA (2007) Ind Eng Chem Res 46:7543–7549
Munera JF, Irusta S, Cornaglia LM, Lombardo EA, Cesar DV, Schmal M (2007) J Catal 245:25–34
Bradford MCJ, Vannice MA (1999) Catal Rev Sci Eng 41:1–42
Graf PO, Mojet BL, van Ommen JG, Lefferts L (2007) Appl Catal A 332:310–317
Wei JM, Iglesia E (2004) J Catal 225:116–127
Maestri M, Vlachos DG, Beretta A, Groppi G, Tronconi E (2008) J Catal 259:211–222
Beretta A, Bruno T, Groppi G, Tavazzi I, Forzatti P (2007) Appl Catal B 70:515–524
Beretta A, Donazzi A, Groppi G, Forzattl P, Dal Santo V, Sordelli L, De Grandi V, Psaro R (2008) Appl Catal B 83:96–109
Hickman DA, Schmidt LD (1993) AIChE J 39:1164–1177
Mhadeshwar AB, Vlachos DG (2005) J Phys Chem B 109:16819–16835
Schwiedernoch R, Tischer S, Correa C, Deutschmann O (2003) Chem Eng Sci 58:633–642
Tavazzi I, Beretta A, Groppi G, Forzatti P (2006) J Catal 241:1–13
Dulaurent O, Chandes K, Bouly C, Bianchi D (2000) J Catal 192:262–272
Belton DN, Schmieg SJ (1988) Surf Sci 202:238–254
Maroto-Valiente A, Rodriguez-Ramos I, Guerrero-Ruiz A (2004) Catal Today 93–5:567–574
Pfnur H, Feulner P, Menzel D (1983) J Chem Phys 79:4613–4623
Jansen MMM, Gracia J, Nieuwenhuys BE, Niemantsverdriet JW (2009) Phys Chem Chem Phys 11:10009–10016
German ED, Sheintuch M (2008) J Phys Chem C 112:14377–14384
Faglioni F, Goddard WA (2005) J Chem Phys 122:14704
Acknowledgements
Our work has been financially supported by the Danish Agency for Science Technology and Innovation. CINF is funded by the Danish National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakobsen, J.G., Jakobsen, M., Chorkendorff, I. et al. Methane Steam Reforming Kinetics for a Rhodium-Based Catalyst. Catal Lett 140, 90–97 (2010). https://doi.org/10.1007/s10562-010-0436-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0436-7