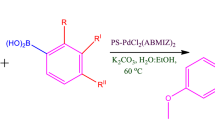
Abstract
Tetrazole functionalized polymer supported palladium complex was synthesized and characterized. The catalytic activity has been evaluated for the room-temperature Suzuki cross-coupling reaction proving an excellent performance in terms of both activity and recyclability. Various aryl bromides were coupled with aryl boronic acids in EtOH/H2O mixed solvents, under air and in presence of 0.5 mol% of the catalyst to afford corresponding cross-coupled products in high yields. Furthermore, the heterogeneous catalyst can be readily recovered by simple filtration and reused for several times only with slightly decrease in its activity.
Graphical Abstract







Similar content being viewed by others
References
Miyaura N, Yamada K, Suzuki A (1979) Tetrahedron Lett 20:3437
Miyaura N, Suzuki A (1995) Chem Rev 95:2457
Diederich J (1998) Metal-catalyzed cross-coupling reaction. Wiley-Interscience, New York
Negishi E, de Meijere A (2002) Handbook of organopalladium chemistry for organic synthesis. Wiley-Interscience, New York
Littke AF, Fu GC (1998) Angew Chem Int Ed 37:3387
Zapf A, Ehrentraut A, Beller M (2000) Angew Chem Int Ed 39:4153
Walker SD, Barder TE, Martinelli JR, Buchwald SL (2004) Angew Chem Int Ed 43:1871
Wolf C, Ekoue-Kovi K (2006) Eur J Org Chem 2006:1917
O’Brien CJ, Kantchev EAB, Valente C, Hadei N, Chass GA, Lough A, Hopkinson AC, Organ MG (2006) Chem Eur J 12:4743
Brendgen T, Frank M, Schatz J (2006) Eur J Org Chem 2006:2378
Dai WM, Zhang Y (2005) Tetrahedron Lett 46:1377
Kovala-Demertzi D, Kourkoumelis N, Derlat K, Michalak J, Andreadaki FJ, Kostas ID (2008) Inorg Chim Acta 361:1562
Chen W, Li R, Han B, Li BJ, Chen YC, Wu Y, Ding LS, Yang D (2006) Eur J Org Chem 2006:1177
Phan NTS, Van Der Sluys M, Jones CW (2006) Adv Synth Catal 348:609
De Vos DE, Dams M, Sels BF, Jacobs PA (2002) Chem Rev 102:3615
Yin L, Liebsher J (2007) Chem Rev 107:133
Maegawa T, Kitamura Y, Sako S, Udzu T, Sakurai A, Tanaka A, Kobayashi Y, Endo K, Bora U, Kurita T, Kozaki A, Monguchi Y, Sajiki H (2007) Chem Eur J 13:5937
Lee D, Kim J, Jun B, Kang H, Park J, Lee Y (2008) Org Lett 10:1609
Zhang J, Zhang W, Wang Y, Zhang M (2008) Adv Synth Catal 350:2065
Trzeciak AM, Mieczynska E, Ziolkowsky J, Bukowski W, Bukowska A, Noworol J, Okal J (2008) New J Chem 32:1124
Yamada YMA, Takeda K, Takahashi H, Ikegami S (2002) Org Lett 4:3371
Yamada YMA, Takeda K, Takahashi H, Ikegami S (2003) J Org Chem 68:7733
Ikegami S, Hamamoto H (2009) Chem Rev 109:583
Mehnert CP, Weaver DW, Ying JY (1998) J Am Chem Soc 120:12289
Dams M, Drijkoningen L, Pauwels B, Van Tendeloo G, De Vos DE, Jacobs PA (2002) J Catal 209:225
Lin KH, Song MP, Cai DM, Hao XQ, Wu YJ (2003) Tetrahedron Lett 44:3955
Varma RS, Naicker KP, Liesen PJ (1999) Tetrahedron Lett 40:2075
Polshettiwar V, Varma RS (2008) Tetrahedron 64:4637
McNamara CA, Dixon MJ, Bradley M (2002) Chem Rev 102:3275
Beletskaya IP, Khokhlov AR, Tarasenko EA, Tyurin VS (2007) J Organomet Chem 692:4402
Polshettiwar V, Molnár Á (2007) Tetrahedron 63:6949
Zhou H, Zhuo GL, Jiang XZ (2006) J Mol Catal A Chem 248:26
Andrushko V, Schwinn D, Tzschucke CC, Michalek F, Horn J, Mossner C, Bannwarth W (2005) Helv Chim Acta 88:936
Gonzalez-Arellano C, Corma A, Iglesias M, Sanchez F (2004) Adv Synth Catal 346:1316
Chang Y, Lv YR, Lu F, Zha F, Lei ZG (2010) J Mol Catal A Chem 320:56
Li Y, Fu X, Gong B, Zou X, Tu X, Chen J (2010) J Mol Catal A Chem 322:55
Byun JW, Lee YS (2004) Tetrahedron Lett 45:1837
Steel PG, Teasdale CWT (2004) Tetrahedron Lett 45:8977
Kim JH, Kim JW, Shokouhimehr M, Lee YS (2005) J Org Chem 70:6714
Kim JW, Kim JH, Lee DH, Lee YS (2006) Tetrahedron Lett 47:4745
Garcia-Martinez JC, Scott RWJ, Crooks RM (2003) J Am Chem Soc 125:11190
van Heerbeek R, Kamer PCJ, van Leeuwen P, Reek JNH (2002) Chem Rev 102:3717
Inada K, Miyaura N (2000) Tetrahedron 56:8661
Parrish CA, Buchwald SL (2001) J Org Chem 66:3820
Glegola K, Framery E, Pietrusiewicz KM, Sinou D (2006) Adv Synth Catal 348:1728
Sommer WJ, Weck M (2006) Adv Synth Catal 348:2101
Bedford RB, Coles SJ, Hursthouse MB, Scordia VJM (2005) Dalton Trans 2005:991
Leyva A, García H, Corma A (2007) Tetrahedron 63:7097
Colacot TJ, Carole WA, Neide BA, Harad A (2008) Organometallics 27:5605
Schweizer S, Becht JM, Drian CL (2010) Tetrahedron 66:765
Ornelas C, Diallo AK, Ruiz J, Astruc D (2009) Adv Synth Catal 351:2147
Gupta AK, Song CH, Oh CH (2004) Tetrahedron Lett 45:4113
Meyer E, Joussef AC, Gallardo H (2003) Synthesis 6:899
Cassol CC, Umpierre AP, Machado G, Wolke SI, Dupont J (2005) J Am Chem Soc 127:3298
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Y., Cai, C. Tetrazole Functionalized Polymer Supported Palladium Complex: An Efficient and Reusable Catalyst for the Room-Temperature Suzuki Cross-Coupling Reaction. Catal Lett 140, 153–159 (2010). https://doi.org/10.1007/s10562-010-0415-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0415-z