Abstract
Nano-MgO a basic catalyst was prepared by solution combustion technique. It was characterized by powder XRD, SEM, BET and TEM analyses. It was used as a catalyst for the study of microwave-assisted N-formylation of various aromatic and alkyl amines with formic acid under solvent-free conditions. Nano-MgO showed excellent catalytic properties and the reactions went to completion, within 1–2 min to give products in high yield (90–98%). The catalyst is recoverable quantitatively and re-cycled with almost consistent activity. This new nano catalyst has the advantages of higher yield, lower cost, reduced environmental hazards, and the procedure is highly convenient.
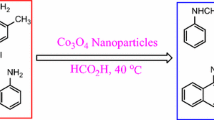
Graphical Abstract
Nano-MgO was prepared and characterized by PXRD, SEM, BET and TEM analyses. It was used for the study of microwave-assisted N-formylation of various amines with formic acid under solvent-free condition.







Similar content being viewed by others
References
Green TW, Wuts PGM (1999) In: Protective groups in organic synthesis, vol 3. Wiley-Interscience, New York
Waki J, Meienhofer J (1977) J Org Chem 42:2019
Schöllkopf U (1977) Angew Chem Int Ed Engl 16:339
Effenberger F, Eichhorn J (1997) Tetrahedron Asymmetr 8:469
Han Y, Cai L (1997) Tetrahedron Lett 38:5423
Jackson A, Meth-Cohn O (1995) J Chem Soc Chem Commun 13:1319
Chen BC, Bednarz MS, Zhao R, Sundeen JE, Chen P, Shen Z, Skoumbourdis AP, Barrish JC (2000) Tetrahedron Lett 41:5453
Kobayashi K, Nagato S, Kawakita M, Morikawa O, Konishi H (1995) Chem Lett 24:575
Kakehi A, Ito S, Hayashi S, Fujii T (1995) Bull Chem Soc Jpn 68:3573
Strazzolini P, Giumanini AG, Cauci S (1990) Tetrahedron 46:1081
Blicke FF, Lu CJJ (1952) J Am Chem Soc 74:3933
Chen FMF, Benoiton NL (1979) Synthesis 709
Yale HL (1971) J Org Chem 36:3238
Neveux M, Bruneau C, Dixneuf PH (1991) J Chem Soc Perkin Trans I 1197
Duczek W, Deutsch J, Vieth S, Niclas HJ (1996) Synthesis 37
Reddy PG, Kumar GDK, Baskaran S (2000) Tetrahedron Lett 41:9149
Sambasivarao K, Manoranjan B, Priti K (2004) Tetrahedron Lett 45:7589
Hill DR, Hasiao CN, Kurukulasuriya R, Wittenberger S (2002) Org Lett 4:111
Szczepankiewicz W, Suwinski J (2000) Chem Heterocycl Compd 36:809
Hosseini-Sarvari M, Sharghi H (2006) J Org Chem 71:6652
Jung SH, Ahn JH, Park SK, Choi JK (2002) Bull Korean Chem Soc 23:149
Biswanath D, Meddeboina K, Balasubramanayam P, Boyapati VD, Nandan KD (2008) Tetrahedron Lett 49:2225
Akbari J, Hekmati M, Sheykhan M, Heydari A (2009) ARKIVOC xi:123
Oliver Kappe C (2008) Chem Soc Rev 37:1127
Bahnemann DW, Kholuiskaya SN, Dillert R, Kulak AI, Kokorin AI (2002) Appl Catal B 36:161
Hosseini-Sarvari M, Sharghi H, Etemad S (2008) Helv Chim Acta 91:715
Mills G, Hoffmann MR (1993) Environ Sci Technol 27:1681
Reddy MBM, Pasha MA (in press) Synth Commun (LSYC-2009-2879)
Pasha MA, Jayashankara VP (2007) Bioorg Med Chem Lett 17:621
Pasha MA, Jayashankara VP (2005) Ultrason Sonochem 12:433
Rama K, Pasha MA (2005) Ultrason Sonochem 12:437
Rama K, Pasha MA (2000) Tetrahedron Lett 41:1073
Nagaraja D, Pasha MA (1999) Tetrahedron Lett 40:7855
Nagappa B, Chandrappa GT (2007) Microporous Mesoporous Mater 106:212
Klug H, Alexander L (1974) In: X-ray diffraction procedures for polycrystalline and amorphous materials, vol 4. Wiley, New York, p. 618
Acknowledgements
G.T.C. gratefully acknowledges the financial support from the Department of Science and Technology, NSTI Phase-IV, Government of India, New Delhi. We also acknowledge the help of Prof. Jai Prakash, Bangalore Institute of Technology, for providing surface area measurement facility and MW reactor. One of the authors M.S. Reddy also wishes to thank Dr. D.N. Sathyanarayana, Professor (Retired) Department of Inorganic and Physical Chemistry, I. I. Sc, Bengaluru, India, for constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, M.B.M., Ashoka, S., Chandrappa, G.T. et al. Nano-MgO: An Efficient Catalyst for the Synthesis of Formamides from Amines and Formic Acid Under MWI. Catal Lett 138, 82–87 (2010). https://doi.org/10.1007/s10562-010-0372-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0372-6