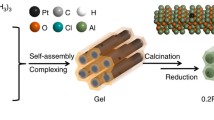
Abstract
Rhodium has been replaced in a synergistic Pt–Rh/γ-Al2O3 catalyst for the reduction of NO with H2 with a less expensive transition metal (Co). Performance in activity and selectivity was still better than Pt alone, thus synergy was again obtained. The nanoparticles in the Pt–Co catalyst were designed and tailored to mimic the microstructure of the synergistic Pt–Rh nanoparticles.
Graphical Abstract
Rhodium has been replaced in a synergistic Pt–Rh/γ-Al2O3 catalyst for the reduction of NO with H2 with a less expensive transition metal (Co), and synergy in activity and selectivity was still obtained. The nanoparticles in the Pt–Co catalyst were designed and tailored to mimic the microstructure of the synergistic Pt–Rh nanoparticles.





Similar content being viewed by others
References
Lakis RE, Lyman CE, Stenger HG (1995) J Catal 154:261
Lakis RE, Cai YP, Stenger HG, Lyman CE (1995) J Catal 154:276
Hu Z, Allen FM, Wan CZ, Heck RM, Steger JJ, Lakis RE, Lyman CE (1998) J Catal 174:13
Dimick PS, Kross JL, Roberts EG, Herman RG, Stenger HG, Lyman CE (2009) Appl Catal B Environ 89:1
Regalbuto JR (ed) (2007) Catalyst preparation: science and engineering. Taylor & Francis, Boca Raton
Lyman CE, Lakis RE, Stenger HG, 2nd Mexican Congress of Electron Microscopy (1994) SSM16
Lyman CE, Lakis RE, Stenger HG (1995) Ultramicroscopy 58:25
Lyman CE, Lakis RE, Stenger HG, Totdal B, Prestvik R (2000) Mikrochim Acta 132:301
Mergler YJ, Nieuwenhuys BE (1997) Appl Catal B Environ 12:95
Mergler YJ, Vanaalst A, Nieuwenhuys BE (1995) Reduct Nitrogen Oxide Emiss 587:196
Mergler YJ, van Aalst S, van Delft J, Nieuwenhuys BE (1996) J Catal 161:310–318
Boix A, Miró EE, Lombardo EA, Bañares MA, Mariscal R, Fierro JLG (2003) J Catal 217:186
Boix AV, Miro EE, Lombardo EA, Mariscal R, Fierro JLG (2004) Appl Catal A Gen 276:197
Gutierrez L, Boix A, Petunchi JO (1998) J Catal 179:179
Gutierrez L, Ribotta A, Boix A, Petunchi J (1996) 11th international congress on catalysis, 40th anniversary, Pts A And B, 631
Ulla MA, Gutierrez L, Lombardo EA, Lónyi F, Valyon J (2004) Appl Catal A Gen 277:227
Jacobs G, Ji Y, Davis BH, Cronauer D, Kropf AJ, Marshall CL (2007) Appl Catal A Gen 333:177
Ko EY, Park ED, Seo KW, Lee HC, Lee D, Kim S (2006) J Nanosci Nanotech 6:3567
Ko EY, Park ED, Lee HC, Lee D, Kim S (2007) Angew Chem Int Ed 46:734
Gajdos M, Hafner J, Eichler A (2005) J Phys Condens Matter 18:41
Okamoto H (2001) J Phase Equilib 22:591
Boer FRD (1988) Cohesion in metals: transition metal alloys. North-Holland, New York
Gajdos M, Hafner J, Eichler A (2005) J Phys Condens Matter 18:13
Bugnard JM, Baudoing-Savois R, Gauthier Y, Hill EK (1993) Surf Sci 281:62
Bugnard JM, Gauthier Y, BaudoingSavois R (1995) Surf Sci 344:42
Mezey LZ, Hofer W (1996) Surf Sci 15:352–354
Ehrhardt C, Gjikaj M, Brockner W (2005) Thermochim Acta 432:36
Borodziński A, Bonarowska M (1997) Langmuir 13:5613
Weisz PB, Prater CD (1954) Adv Catal Relat Subj 6:143
Weisz PB (1957) Z Phys Chem (Frankfurt am Main) 11:1
Froment GF, Bischoff KB (1990) Chemical reactor analysis and design. Wiley, Hoboken
Mukherjee S, Vannice MA (2006) J Catal 243:108
Acknowledgments
The authors thank David Ackland for assistance acquiring the catalyst images on the JEOL 2200 FS. Funding for this project was provided by the National Science Foundation (Grant DMR-0506705).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimick, P.S., Herman, R.G. & Lyman, C.E. Logical Design for Replacement of Rh with Co in a Synergistic Catalyst for the Reduction of NO with H2 . Catal Lett 135, 33–40 (2010). https://doi.org/10.1007/s10562-010-0273-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0273-8