Abstract
Ca1−x La x NiAl11O19−δ(O ≤ x≤1) hexaaluminate oxydes were synthesized starting from nitrate salts of Ca, La, Ni and Al precipitated by citric acid. After calcination they were used as catalysts precursors in dry reforming of methane to synthesis gas at atmospheric pressure (600–800 °C) with a mixture of CH4/CO2/Ar:1/1/3. The solids were characterized by X-ray diffraction (XRD), BET surface area, temperature programmed reaction and oxidation (TPO) and by X-ray photoelectron spectroscopy. XRD analysis shows a pure hexaluminate phase as soon as a part of calcium has been substituted by lanthanum. After H2 reduction and after reactivity test, Ni metal characterized by XRD is responsible of the high activity (equilibrium conversion near 100% at 800 °C). Ni hexaaluminate shows a remarkable high stability (more than 300 h test) probably due to the low formation of surface carbon (TPO).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Natural gas is recognized as one of the fuel of the near future due to the worldwide proven reserve [1]. This situation has produced a growing interest in the development of technologies able to transform natural gas to liquid energy through the syngas. Dry reforming (CH4 + CO2 → 2CO + 2H2 ΔH298 = 247 kJ mol−1) produces syngas with a H2/CO ratio near 1 favourable for Fischer-Tropsch or hydrocarbonylation reactions. Dry reforming could also have environmental implication by the thermochemical valorisation of CO2. However, one disadvantage of dry reforming is the accumulation of carbon by methane cracking or CO disproportion over catalytic surface. Coke deposition is strongly influenced by the type of metal used. Noble metals present lower level of coke than nickel [2], however, Ni is generally used because of it low cost and availability [3]. Carbon formation decreases with high nickel dispersion, basic supports, redox promoters [4–6] and also if catalyst presents a strong interaction between nickel and the support. This last feature is obtained when nickel is initially present in a defined structure like perovskite [7], spinel [8], fluorite [9] or in a solid solution (NiO–MgO) [5]. With these structures, metal reduction temperature is increased at a temperature near of the reaction temperature and highly dispersed metallic nanoparticles are produced. The presence of alkali earth or rare earth favours carbonates or oxycarbonates formation and it is well documented that such species react with carbon with CO formation [10].
Hexaaluminates materials ANiAl11O19−δ (A = La, Ca, Ba, Sr) are defined structures, they contain alkali or rare earth oxides and α-alumina has been indicated as preventing coke formation [11]. Consequently they are potential candidates to dry reforming. In fact, LaNi y Al12−yO19−δ (y = 0.3; 0.6; 0.9 and 1) in which Ni ion is inlayed in hexaaluminate lattice to substitute part of Al ions are of interest and high resistance to carbon deposition and long term stability (300 h) were observed for La0.8Pr0.2NiAl11O19−δ [12, 13]. In the present study, a series of CaLaNi hexaaluminate were prepared by substitution of Ca by La, characterized and tested in dry reforming of methane in order to investigate the influence of the presence of La in the CaNi hexaaluminate structure on the catalytic dry reforming activity and on the carbon deposition resistance.
2 Experimental
2.1 Catalyst Preparation
The hexaaluminate Ca1−x La x NiAl11O19−δ(O ≤ x ≤ 1) were prepared as follows: calcium, lanthanum, nickel and aluminium nitrates were dissolved separately in distilled water mixed together to obtain a molar ratio Ca/La/Ni/Al equal to (1 − x)/x/1/11, respectively. The aqueous solution was slowly added at room temperature to citric acid and heated at 60 °C under stirring to remove excess of water and until formation of a vitreous gel. The gel was then dried at 110 °C overnight and calcined 2 h at 500 °C then 8 h to 1,100 °C.
2.2 Catalyst Characterization
XRD patterns have been obtained using SIEMENS D500 difractometer (CuKα radiation : λ = 1.5418 Å). The obtained phases were determined using fishier ASTM programs (American Society for Testing Material) type DATA FILE PDF Diffraction 2000.
BET surface areas were determined by nitrogen adsorption using Coulter SA 3100 apparatus. Low specific surface areas were obtained (1–6 m2 gcat−1, for all samples). Binding energy and chemical composition of surface were measured by XPS (Thermo VG Multilab 2000 using AlKa radiation).
The reducibility of Ni hexaaluminates was studied by TPR and carbon entities formation was determined by TPO as follow: 0.5 g of catalyst was embedded in a quartz reactor (inner diameter 6.6 mm). After purge by argon, the catalyst was heated from room temperature to 900 °C (15 °C min−1) in a H2/Ar mixture (2/50 mL min−1) for TPR and in a 5/45 mL min−1 O2/Ar mixture for TPO measurements. In both cases, effluent gas composition was analyzed using TCD gas chromatograph.
2.3 Catalytic Activity Test
CH4/CO2 activities were performed in a fixed-bed quartz reactor under atmospheric pressure. 0.05 g of catalyst was first reduced in pure H2 flow (1 L h−1) at 900 °C, 1 h then purged by Ar and cooled at room temperature for 15 min. Dry reforming gas (CH4/CO2/Ar:1/1/3; total flow 20 L h−1 gcat−1) was then admitted at 800 °C temperature. The catalytic activities were investigated between 500 and 900 °C. The reactants (CH4 and CO2) and products (CO, CO2, H2) were analyzed on line using TCD gas chromatograph equipped with a Carbosieve column.
3 Results and Discussion
3.1 XRD
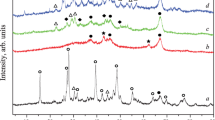
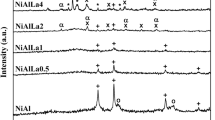
Figure 1 shows the XRD patterns of Ca1−x La x NiAl11O19−δ hexaaluminates (0 ≤ x ≤ 1) after calcination at 1,100 °C. For LaNiAl11O19−δ a pure hexaaluminate phase is formed with characteristic diffraction peaks at 36.08; 34.02 and 32.06 (2θ). For CaNiAl11O19−δ, we characterize hexaaluminate with low cristallinity, α-Al2O3 and NiAl2O4 spinel diffraction peaks localized at 25.3 (2θ) and 37.1 (2θ). In accordance with the literature [14] a A3+ cation can promote the formation of Ni hexaaluminate more effectively than A2+ cation and as soon few amounts of La3+ is added (x = 0.1) Ni hexaluminate structure is well crystallized.
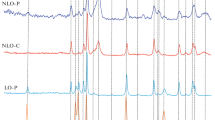
As shown in Figs. 2 and 3, the Ni hexaaluminate structure is always present after reduction (until 900 °C) and after dry reforming. After TPR, it is observed at 44.5 (2θ) the characteristic peak of Ni metal. In fact, only a part of the Ni2+ of the hexaluminate structure has been reduced to Ni° and this will change slightly with the La3+ content. In Fig. 3, Ni metal is always present at 44.5 (2θ) and a characteristic peak of carbon at 26.8° (2θ) is evidenced. The intensity of this peak depends of the La content and carbon deposition seems particularly important on Ca0.7La x.3NiAl11O19−δ.
3.2 TPR
TPR profiles of La1−x La x NiAl11O19−δ catalysts are reported Fig. 4. For all the catalysts, reducibility of Ni is only obtained at very high temperature (>800 °C). This clearly indicates that it will be absolutely necessary to reduce all the catalysts before reactivity tests. Maximum of reduction is obtained at nearly 900 °C compared to 400 °C for NiO [11]. The temperature of the maximum does not change a lot.
However, Ca0.5La0.5NiAl11O19−δ is reduced at the lowest temperature. The surface reduction changes also with the content of La. From the evaluation surface area of the hydrogen consumption during TPR, reducibility of Ca0.5La0.5NiAl11O19−δ is the highest and has been evaluated at 94% for the nickel. The reduction of the other catalysts has been evaluated between 88 and 92%.
3.3 XPS
The binding energy of the elements of the Ni hexaaluminate is given Table 1. The binding energy of each element is not affected by the Ca substitution by La. For nickel, Ni2p3/2 binding energy of 855.5 eV indicates the presence of nickel into the hexaaluminate lattice in the Ni2+ state [15]. La, Ca, Al ions are, respectively, in the 3+; 2+ and 3+ oxidation states. Table 2 shows the chemical composition at the catalytic surface.
Surface concentrations of La and of Ni are largely lower than nominal composition. This is in accordance with literature data [15, 16]. It is suggested that segregation of Ni is driven by difference in binding energies between the respective elements [17]. The Ni surface concentration increases with the x value for the same reasons. Xu et al. [16] have suggested that the surface Ni concentration correlates with both size and valence of minor cation.
4 Catalytic Activity of Ca1−x La x NiAl11O19−δ Hexaaluminates in Dry Reforming of Methane
The activity of reduced Ca1−x La x NiAl11O19−δ is given Fig. 5 between 500 and 900 °C. No clear correlation was observed between CO2 or CH4 reactivity and Ca/La ratio. Even with the low amount of Ni present at the surface (XPS results), all the catalysts present a high activity following thermodynamic data except for Ca = 0.3 and 0.7. Activity is the highest for Ca0.5La0.5NiAl4O19−δ which is not the catalyst with the highest content of surface nickel but the catalyst with the highest reducibility (see Table 1). The H2/CO ratio is always near 1 except for Ca = 0.3. Formation of carbonaceous species is given (Fig. 6) and characterized by TPO. All the catalysts present three main peaks: one is located between 540 and 590 °C. It corresponds to surface carbon deposited as nanotubes on the catalyst. The second one is located between 440 and 455 °C and could be tentatively attributed to carbon located at the vicinity rare earth at of metal and rare earth [18]. It appears like a shoulder of the first one. The third one is located between 330 and 370 °C. Surface area is increasing with the percentage of lanthanum. It could be attributed at the presence of surface carbonates.
Finally the stability of the catalysts has been investigated at 800 °C during 6 h (Fig. 7), again Ca0.5La0.5NiAl11O19−δ is the most active for CH4 and CO2 conversion, Ca = 0.3 given the lowest activity. It should be noticed that in absence of La, the CaNiAl catalyst lost quickly some activity.
5 Conclusion
A series of substituted Ca1−x La x NiAl11O19−δ hexaaluminates were prepared using citric acid method. All the obtained solids have low surface area after calcination at 1,100 °C. As soon lanthanum is present, the formation of hexaaluminate has been confirmed by XRD. After reduction, content of Ni at the catalytic surface is low. However, all the catalysts and more specifically Ca0.5La0.5NiAl11O19−δ which has the highest percentage of Ni reduced at the surface (XPS) shows high activity for syngas production but also high stability for the reaction (300 h).
References
BP Statistical Review of World Energy (2008)
Hu YH, Ruckenstein E, Bruce CG, Helmut K (2004) Adv Catal 48:297
Basile F, Fornasari G, Trifiro F, Vaccari A (2002) Catal Today 77:215
Ruckenstein E, Hu YH (1995) Appl Catal A 133:149
Ruckenstein E, Hu YH (1999) Appl Catal A 183:85
Provendier H, Petit C, Estournes C, Libs S, Kiennemann A (1999) Appl Catal A 180:163
Djaidja A, Libs S, Kiennemann A, Barama A (2006) Catal Today 113:194
Sahli N, Petit C, Roger AC, Kiennemann A, Libs S, Bettahar MM (2006) Catal Today 113:187
Romera-Saria F, Vargas JC, Roger AC, Kiennemann A (2008) Catal Today 133–135:149
Gallego GS, Batiot-Dupeyrat C, Barrault J, Florez E, Mondragon F (2008) Appl Catal A 334:251
Rostrupp-Nielsen JR, Schested J, Norskov JK (2002) Adv Catal 47:65
Liu Y, Xu Z, Li D, Chen T, Zhou G, Li W, Bi Y, Zhen K (2002) Kinet Catal 43:522
Liu Y, Cheng T, Li D, Jiang P, Wang J, Li W, Bi Y, Zhen K (2003) Catal Lett 85:101
Li S, Liu H, Yan L, Wang X (2007) Catal Comm 8:237
Xu Z, Zhen M, Bi Y, Zhen K (2000) Appl Catal A 198:267
Xu Z, Zhen M, Bi Y, Zhen K (2000) Catal Lett 64:157
Gardner TH, Shekhawat D, Berry DA, Smith MW, Salazar M, Kugler EL (2007) Appl Catal A Gen 323:1
Zhu T, Flytzani-Stephanopoulos M (2001) Appl. Cat. A 208:403
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ikkour, K., Sellam, D., Kiennemann, A. et al. Activity of Ni Substituted Ca-La-hexaaluminate Catalyst in Dry Reforming of Methane. Catal Lett 132, 213–217 (2009). https://doi.org/10.1007/s10562-009-0094-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0094-9