Abstract
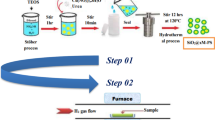
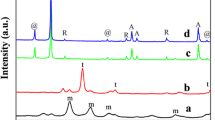
An efficient, mild and practical method for the reduction of aromatic nitro compounds using Ni/SiO2 as catalyst is reported. The catalyst has been prepared and characterized by XRD, FT-IR, TEM, SEM successfully. The Ni loading is about 55 wt.%. The prominent merit of the Ni/SiO2 catalyst passivated with a gas mixture is that it can be stored safely in air below 423 K and needs no activation before use. In the catalytic test, Ni/SiO2 is found to be a highly efficient and reusable catalyst for the reduction of various aromatic nitro compounds. This catalyst is applicable for the preparation of various aromatic amines with different groups, the conversion and selectivity are almost up to 100%. Moreover, it is inexpensive, can be prepared and scaled up easily.





Similar content being viewed by others
References
Booth G (2002) Ullmanns encyclopedia of industrial chemistry. Wiley-VCH Verlag, Weinheim
(a) Figueras F, Coq B (2001) J Mol Catal A: Chem 173:117–134; (b) Figueras F, Coq B (2001) J Mol Catal A: Chem 173:223–230
Mitchell SC, Waring RH (2000) Ullmanns encyclopedia of industrial chemistry. Wiley-VCH Verlag, Weinheim
Bechamp AJ (1854) Anal Chim Phys 42:140
Lakshmi Kantam M, Bandyopadhyay T, Rahman A, Mahender Reddy N, Choudary BM (1998) J Mol Catal A: Chem 133:293–295
Xu S, Xi X, Shi J, Cao S (2000) J Mol Catal A: Chem 160:287–292
Chen Y, Qiu J, Wang X, Xiu J (2006) J Catal 242:227–230
Du Y, Chen H, Chen R, Xu N (2004) Appl Catal A: Gen 277:259–264
Joseph T, Vijay Kumar K, Ramaswamy AV, Halligudi SB (2007) Catal Commun 8:629–634
(a) Cardenas-Lizana F, Gomez-Quero S, Keane MA (2008) Catal Commun 9:475–481 (b) Cardenas-Lizana F, Gomez-Quero S, Keane MA (2008) Appl Catal A: Gen 334:199–206
Bovkun TT, Grayevsky M, Sasson Y, Bluma J (2007) J Mol Catal A: Chem 270:171–176
Shen J-H, Chen Y-W (2007) J Mol Catal A: Chem 273:265–276
Basu B, Das S, Das P, Nanda AK (2005) Tetrahedron Lett 46:8591–8593
Murthy KV, Patterson PM, Jacobs G, Davis BH, Keane MA (2004) J Catal 223:74–85
Xi X, Liu Y, Shi J, Cao S (2003) J Mol Catal A: Chem 192:1–7
Keum Y-S, Li QX (2004) Chemosphere 54:255–263
Corma A, Serna P (2006) Science 313:332
Corma A, Concepcion P, Serna P (2007) Angew Chem Int Ed 46:7266 –7269
Raja R, Golovko VB, Thomas JM, Berenguer-Murcia A, Zhou W, Xie S, Johnson BFG (2005) Chem Commun 2026–2028
He D, Shi H, Wu Y, Xu B-Q (2007) Green Chem 9:849–851
Kulkarni AS, Jayaram RV (2003) Appl Catal A: Gen 252:225–230
Kulkarni AS, Jayaram RV (2004) J Mol Catal A: Chem 223:107–110
Mohapatra SK, Sonavane SU, Jayaram RV, Selvam P (2002) Tetrahedron Lett 43(47): 8527–8529
Mohapatra SK, Sonavane SU, Jayaram RV, Selvam P (2002) Org Lett 4(24): 4297–4300
Benz M, Prins R (1999) Appl Catal A: Gen 183:325–333
Abiraj K, Srinivasa GR, Gowda DC (2005) Aust J Chem 58(2):149–151
Abiraj K, Srinivasa GR, Gowda DC (2005) Synth Commun 35(2):223–230
Jyothi TM, Rajagopal R, Sreekumar K, Talawar MB, Sugunanb S, Rao BS (1999) J Chem Res (S):674–675
Joshi MV, Mukesh D (1997) J Catal 168:273–277
Mdleleni MM, Rinker RG, Ford PC (2003) J Mol Catal A: Chem 204–205:125–131
Nomura K (1995) J Mol Catal A: Chem 95:203–210
Liu X, Lu S (2004) J Mol Catal A: Chem 212:127–130
Basu MK, Becker FF, Banik BK (2000) Tetrahedron Lett 41:5603–5606
Lauwiner M, Rys P, Wissmann J (1998) Appl Catal A: Gel 172:141–148
Pogorelić I, Filipan-Litvić M, Merkaš S, Ljubić G, Cepanec I, Litvić M (2007) J Mol Catal A: Chem 274:202–207
Gowda S, Channe Gowda D (2002) Tetrahedron 58:2211–2213
Khan FA, Dash J, Sudheer Ch, Gupta RK (2003) Tetrahedron Lett 44:7783–7787
Islam SM, Saha CR (2004) J Mol Catal A: Chem 212:131–140
Dileep Kumar JS, Ho MM, Toyokuni T (2001) Tetrahedron Lett 42:5601–5603
McLaughlin MA, Barnes DM (2006) Tetrahedron Lett 47:9095–9097
Deka DC, Kakati HS (2006) J Chem Res 4:223–224
Zhang C-R, Wang Y-L (2006) Indian J Chem Sect B: Org Chem Incl Med Chem 45(2):510–511
Rahaim RJJ, Maleczka REJ (2005) Org Lett 7(22):5087–5090
Sarmah P, Dutta DK (2003) J Chem Res Synop 4:236–237
Banik BK, Mukhopadhyay C, Venkatraman MS, Becker FF (1998) Tetrahedron Lett 39:7243–7246
Banik BK, Suhendra M, Banik I, Becker FF (2000) Synthetic Commun 30(20):3745–3754
Desai DG, Swami SS, Hapase SB (1999) Synthetic Commun 29(6):1033–1036
Desai DG, Swami SS, Dabhade SK, Ghagare MG (2001) Synthetic Commun, 31(8):1249–1251
Kumbhar PS, Sanchez-Valente J, Millet JMM, Figueras F (2000) J Catal 191:467–473
(a) Aiello R, Fiscus JE, zur Loye H-C, Amiridis MD (2000) Appl Catal A: Gen 192:227–234 (b) Takahashi R, Sato S, Sodesawa T, Kato M, Takenaka S, Yoshida S (2001) J Catal 204:259–271 (c) Ennas G, Mei A, Musinu A, Piccaluga G, Pinna G, Solinas S (1998) J Non-Cryst Solids 232–234:587–593 (d) Matsumura Y, Tode N, Yazawa T, Haruta M (1995) J Mol Catal A: Chem 99:183–185
(a) Xiong H, Tang S, Tang H, Zou P (2008) Carbohyd Polym 71:263–268 (b) Wu KH, Chang YC, Wang GP (2004) J Magn Magn Mater 269:150–155 (c) Song CF, Lu MK, Gu F, Liu SW, Wang SF, Xu D, Yuan DR (2003) Inorg Chem Commun 6(5):523–526 (d) Phan NTS, Jones CW (2006) J Mol Catal A: Chem 253:123–131
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, Y., Ma, K., Wang, H. et al. A Green Reduction of Aromatic Nitro Compounds to Aromatic Amines Over a Novel Ni/SiO2 Catalyst Passivated with a Gas Mixture. Catal Lett 124, 268–276 (2008). https://doi.org/10.1007/s10562-008-9452-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9452-2