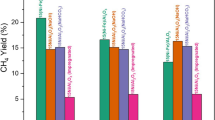
We have developed a new Pt–Fe/mordenite (Pt–Fe/M) catalyst which shows remarkably high activity and selectivity for the oxidation of CO in H2-rich gas compared with Pt/M. In the present work, to understand the role and structure of Pt and Fe in the Pt–Fe/M catalyst, the states of metallic components in ion-exchanged, H2 pre-treated and post-PROX (preferential oxidation of CO) samples have been studied by means of XAFS. It was confirmed that Pt forms the metallic clusters after H2 pretreatment or the PROX experiment, whereas a large part of Fe exists as oxides even after the H2 treatment. At post-analysis of the catalysts used for the PROX experiment, an increase in coordination number of Fe–O was observed. Pt clusters in the Pt–Fe(2:1 weight ratio)/M catalyst, which showed the highest PROX performance, were found to have a different electronic structure from the other catalysts. Additionally, preferential CO adsorption onto Pt sites at Pt–Fe/M was clearly demonstrated by infrared spectroscopy analysis in a stream of 1% CO containing H2. Based on these results, the superior PROX mechanism was discussed.
Similar content being viewed by others
References
R.A. Lemons (1990) J. Power Sources 29 251
H. Igarashi T. Fujino M. Watanabe (1995) J. Electroanal. Chem. 391 119 Occurrence Handle10.1016/0022-0728(95)03914-3
M. Watanabe S. Motoo (1975) J. Electroanal. Chem. 60 275 Occurrence Handle10.1016/0368-1874(75)87031-6
M.L. Brown SuffixJr. A.W. Green (1960) Ind. Eng. Chem. 52 841 Occurrence Handle10.1021/ie50610a025
P.V. Snytnikov V.A. Sobyanin V.D. Belyaev P.G. Tsyrulnikov N.B. Shitova D.A. Shlyapin (2003) Appl. Catal. A 239 149 Occurrence Handle10.1016/S0926-860X(02)00382-4
M. Watanabe, H. Uchida, H. Igarashi and M. Suzuki, Chem. Lett. (1995) 21
H. Igarashi, H. Uchida and M. Watanabe, Chem. Lett. (2000) 1262
H. Igarashi H. Uchida M. Watanabe (2001) Stud. Surf. Catal. 132 953
M. Watanabe H. Uchida K. Ohkubo H. Igarashi (2003) Appl. Catal. B 46 595 Occurrence Handle10.1016/S0926-3373(03)00322-9
M. Newville P. Livins Y. Yacoby E.A. Stern J.J. Rehr (1993) Phys. Rev. B 47 14126 Occurrence Handle10.1103/PhysRevB.47.14126
M. Newville B. Ravel D. Haskel J.J. Rehr E.A. Stern Y. Yacoby (1995) Physica B208-209 154
A.L. Ankudinov B. Ravel J.J. Rehr S.D. Conradson (1998) Phys. Rev. B 58 7565 Occurrence Handle10.1103/PhysRevB.58.7565
D. Exner N. Jaeger K. Moller G. Schulz-Ekloff (1982) J. Chem. Soc. Faraday Trans. 1 78 3537 Occurrence Handle10.1039/f19827803537
J. Graaf Particlede A.J. Dillen Particlevan K.P. Jong Particlede D.C. Koningsberger (2001) J. Catal. 203 307 Occurrence Handle10.1006/jcat.2001.3337
W.M. Meier (1961) Z. Kristallogr. 115 439
G.F. Cabeza P. Legare N.J. Castellani (2000) Surf. Sci. 465 286 Occurrence Handle10.1016/S0039-6028(00)00715-9
P. Pillonel S. Derrouiche A. Bourane F. Gaillard P. Vernoux D. Bianchi (2005) Appl. Catal. A 278 223 Occurrence Handle10.1016/j.apcata.2004.10.001
R. Cataliotti A. Foffani L. Marchetti (1971) Inorg. Chem. 10 1594 Occurrence Handle10.1021/ic50102a010
T. Tanabe R. Buckmaster T. Ishibashi T. Wadayama A. Hatta (2001) Surf. Sci. 472 1 Occurrence Handle10.1016/S0039-6028(00)00931-6
M. Watanabe H. Igarashi T. Fujino (1999) Electrochemistry 67 1194
H. Igarashi T. Fujino Y. Zhu H. Uchida M. Watanabe (2001) Phys. Chem. Chem. Phys. 3 306 Occurrence Handle10.1039/b007768m
S.H. Oh R.M. Sinkevitch (1993) J. Catal. 142 254 Occurrence Handle10.1006/jcat.1993.1205
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kotobuki, M., Shido, T., Tada, M. et al. XAFS Characterization of Pt–Fe/zeolite Catalysts for Preferential Oxidation of CO in Hydrogen Fuel Gases. Catal Lett 103, 263–269 (2005). https://doi.org/10.1007/s10562-005-7163-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-005-7163-5