Abstract
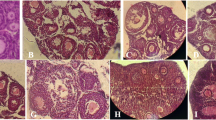
Most protocols to cryopreserve ovarian tissue utilize the permeable cryoprotective agents (CPAs) in 1.5 M concentration. However the issues related to the ability to use higher concentrations of CPAs have remained open. The research aim was to assess the efficiency of media containing osmotically active sugars (sucrose, mannitol) at stepwise adding/removing of 3 M dimethylsulphoxide (DMSO) and propanediol (PROH) on ovarian tissue integrity. After the CPAs adding/removing the ovarian tissue injury was histologically examined, as well as the oocyte volume in tissue structure was assessed. It has been found, that after adding/removing of PROH and DMSO solutions the maximum amount of normal follicles made 67–93% when using Dulbecco’s Modified Eagle Medium (DMEM) with 200 mM sucrose. Assessment of tissue damage after adding/removing of CPAs has demonstrated that the percentage of normal follicles was 83–87% using DMSO in presence of both sucrose and mannitol as the dilution media components. While removing PROH the level of follicles preservation was 2.5× higher when using mannitol compared with sucrose. Our results indicate that the ovarian tissue injury was minimal during adding 3 M CPAs using DMEM, containing sucrose and following application of mannitol at removing both DMSO and PROH.





Similar content being viewed by others
References
Amorim CA, Dolmans MM, David A et al (2012) Vitrification and xenografting of human ovarian tissue. Fertil Steril 98(5):1291–1298
Baird DT, Webb R, Campbel BK, Harknews LM, Gosden RG (1999) Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 °C. Endocrinology 140:462–471
Candy CJ, Wood MJ, Whittinghan DG (1997) Effect of cryoprotectants on the survival of follicles in frozen mouse ovaries. J Reprod Fertil 110:11–19
Cox SL, Shaw J, Jenkin G (1996) Transplantation of cryopreserved fetal ovarian tissue to adult recipients inmice. J Reprod Fertil 107:315–322
Demeestere I, Simon P, Buxant F et al (2006) Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod 21:2010–2014
Demeestere I, Simon P, Emiliani S et al (2009) Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod 15(6):649–665
Demirci B, Lornage J, Salle B et al (2001) Follicular viability and morphology of sheep ovaries after exposure to cryoprotectant and cryopreservation with different freezing protocols. Fertil Steril 75:754–762
Demirci B, Salle B, Frappart L, Franck M, Guerin JF, Lornage J (2002) Morphological alterations and DNA fragmentation in oocytes from primordial and primary follicles after freezing–thawing of ovarian cortex in sheep. Fertil Steril 77:595–600
Donnez J, Dolmans MM (2010) Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol 53:787–796
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard P, Squifflet J, Martinez-Madrid B, Van Langendonckt A (2004) Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364:1405–1410
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM (2011) Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med 43:437–450
Eyden B, Radford J, Shalet SM, Thomas N, Brison DR, Lieberman BA (2004) Ultrastructural preservation of ovarian cortical tissue cryopreserved in dimethylsulfoxide for subsequent transplantation into young female cancer patients. Ultrastruct Pathol 28:239–245
Fabbri R, Pasquinelli G, Keane D, Magnani V, Paradisi R, Venturoli S (2010) Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reprod Biomed Online 21:819–828
Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O (2003) Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod 18:2420–2428
Hsueh AJ, Eisenhauer K, Chun SY, Hsu SY, Billig H (1996) Gonadal cell apoptosis. Recent Prog Horm Res 51:433–456
Isachenko E, Isachenko V, Nawroth F, Rahimi G, Kreienberg R, Reinsberg J, Weiss J (2008) Human ovarian tissue preservation: is vitrification acceptable method for assisted reproduction? Cryoletters 29:301–314
Karibe H, Zarow GJ, Weinstein PR (1995) Use of mild intraischemic hypothermia versus mannitol to reduce infarct size after temporary middle cerebral artery occlusion in rats. J Neurosurg 83:93–98
Karlsson JO, Younis AI, Chan AW, Gould KG, Eroglu A (2009) Permeability of the rhesus monkey oocyte membrane to water and common cryoprotectants. Mol Reprod Dev 76:321–333
Kim SS, Yang HW, Kang HG et al (2004) Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril 82:679–685
Matos MHT, Andrade ER, Lucci CM et al (2004) Morphological and ultrastructural analysis of sheep primordial follicles preserved in 0.9% saline solution and TCM 199. Theriogenology 62:65–80
Mazur P, Schneider U (1986) Osmotic responses of preimplantation mouse and bovine embryos and their cryobiological implications. Cell Biophys 8:259–285
Mierow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Dor J (2005) Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy (letter). N Engl J Med 353:318–321
Mierow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, Dor J (2007) Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril 87:418
Neto V, Buff S, Lornage J et al (2008) Effects of different freezing parameters on the morphology and viability of preantral follicles after cryopreservation of doe rabbit ovarian tissue. Fertil Steril 89(3):1348–1356
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R (1996) Low temperature storage and grafting of human ovarian tissue. Hum Reprod 11:1487–1491
Newton H, Fisher J, Arnold JRPRG et al (1998) Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod 13:376–380
Newton H, Pegg DE, Barrass R, Gosden RG (1999) Osmotically inactive volume, hydraulic conductivity, and permeability to dimethyl sulphoxide of human mature oocytes. J Reprod Fertil 117:23–27
Oktay K, Newton H, Aubard Y, Salha O, Gosden RG (1998) Cryopreservation of immature human oocytesand ovarian tissue: an emerging technology? Fertil Steril 354:1–7
Protocol of amendment to the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes, No. 170, Strasbourg, 22.VI.1998
Rodrigues APR, Amorim CA, Costa SHF et al (2004) Cryopreservation of caprine ovarian tissue using dimethylsulphoxide and propanediol. Anim Reprod Sci 84:211–227
Sağsöz N, Kisa U, Apan A (2002) Ischaemia–reperfusion injury of rat ovary and the effects of vitamin C, mannitol and verapamil. Hum Reprod 17:2972–2976
Schubert B, Canis M, Darcha C, Artonne Ch, Pouly JL, Dechelotte P, Boucher D, Grizard G (2005) Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod 20:1786–1792
Songsasen N, Ratterree MS, VandeVoort CA et al (2002) Permeability characteristics and osmotic sensitivity of rhesus monkey (Macaca mulatta) oocytes. Hum Reprod 17:1875–1884
Suzuki J, Imaizumi S, Kayama T, Yoshimoto T (1985) Chemiluminescence in hypoxic brain—the second report: cerebral protective effect of mannitol, vitamin E and glucocorticoid. Stroke 16:695–700
Wang LH, Mullen SF, Li Y, Zhong JQ, Crister JK, Chen ZJ (2009) Morphological and apoptotic comparison of primordial and primary follicles in cryopreserved human ovarian tissue. Reprod Domest Anim 44:879–883
Woods EJ, Benson JD, Agca Y, Critsera JK (2004) Fundamental cryobiology of reproductive cells and tissues. Cryobiology 48:146–156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiroshka, V., Trutaieva, I. & Bondarenko, T. Efficiency of mannitol-supplemented medium during adding/removing ovarian tissue with penetrating cryoprotective agents. Cell Tissue Bank 19, 123–132 (2018). https://doi.org/10.1007/s10561-017-9623-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9623-8