Abstract
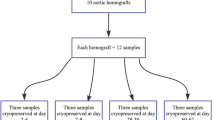
The objective of this study was to design and test a protocol for the validation of banking methodologies for arterial allografts. A series of in vitro biomechanical and biological assessments were derived, and applied to paired fresh and banked femoral arteries. The ultimate tensile stress and strain, suture pullout stress and strain, expansion/rupture under hydrostatic pressure, histological structure and biocompatibility properties of disinfected and cryopreserved femoral arteries were compared to those of fresh controls. No significant differences were detected in any of the test criteria. This validation protocol provides an effective means of testing and validating banking protocols for arterial allografts.







Similar content being viewed by others
References
Angell WW, Angell JD, Oury JH, Lamberti JJ, Grehl TM (1987) Long term follow up of viable frozen aortic homografts: a viable homograft valve bank. J Thorac Cardiovasc Surg 93:815–822
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124
Chiesa R, Astore D, Piccolo G, Melissano G et al (1998) Fresh and cryopreserved arterial homografts in the treatment of prosthetic graft infections: experience of the Italian Collaborative Vascular Homograft Group. Ann Vasc Surg 12(5):457–462
Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. OJEU 7.4.2004 L102/48-58
Hunt CJ, Song YC, Bateson EA, Pegg DE (1994) Fractures in cryopreserved arteries. Cryobiology 31:506–515
ISO 10993 (5) (1998b) Biological evaluation of medical devices—tests for in vitro cytotoxicity. International Organization for Standardization
ISO 7198 (1998a) Cardiovascular implants—tubular vascular prostheses. International Organization for Standardization
Jashari R, Van Hoeck B, Tabaku M, Vanderkelen A (2004) Banking of the human heart valves and the arteries at the European homograft bank (EHB)–overview of a 14-year activity in this International Association in Brussels. Cell Tissue Bank 5(4):239–251
Kieffer E, Bahnini A, Koskas F, Ruotolo C, Le Blevec D, Plissonnier D (1993) In situ allograft replacement of infected infrarenal aortic prosthetic grafts: results in forty-three patients. J Vasc Surg 17(2):349–355
Kieffer E, Gomes D, Chiche L, Fléron MH, Koskas F, Bahnini A (2004) Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg 39(5):1009–1017
Leeming JP, Lovering AM, Hunt CJ (2005) Residual antibiotics in allograft heart valve tissue samples following antibiotic disinfection. J Hosp Infect 60(3):231–234
Mestres CA, Mulet J, Pomar JL (1995) Large calibre cryopreserved arterial allografts in vascular reconstructive operations: early experience. Ann Thorac Surg 60:s105–s107
Morrison TM, Choi G, Zarins CK, Taylor CA (2009) Circumferential and longitudinal cyclic strain of the human thoracic aorta: age-related changes. J Vasc Surg 49(4):1029–1036
Mossman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Pegg DE, Wusteman MC, Boylan S (1997) Fractures in cryopreserved elastic arteries. Cryobiology 34:183–192
Rules and guidance for pharmaceutical manufacturers and distributors, 7th edition 2007, Pharmaceutical press, London
Sokal RR, Rohlf FJ (eds) (1995) Biometry, 3rd edition, W. H Freeman and Co., New York, p 248
Szilagyi DE, Rodriguez FJ, Smith RF, Elliot JP (1970) Late fate of arterial allografts: observations 6–15 years after implantation. Arch Surg 101:721–733
Acknowledgments
We would like to thank Dr. Anders Vanderkelen, Dr. Ramadan Jashari and their colleagues at the European Homograft Bank for their advice and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lomas, R.J., Dodd, P.D.F., Rooney, P. et al. A standardised protocol for the validation of banking methodologies for arterial allografts. Cell Tissue Bank 14, 495–503 (2013). https://doi.org/10.1007/s10561-012-9346-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-012-9346-9