Abstract
Purpose
This study was designed to investigate the impact of single-nucleotide polymorphism-encoded cytochrome P450 enzymes (CYP3A4/5) on clinical outcomes of rivaroxaban in patients with non-valvular atrial fibrillation (NVAF) based on pharmacokinetics and pharmacodynamics (PK/PD) aspects.
Method
A prospective study enrolling 165 rivaroxaban-treated patients with NVAF was conducted. Genotyping of CYP3A4 (rs2242480, rs2246709, rs3735451, and rs4646440) and CYP3A5 (rs776746) was performed to explore their impact on the trough plasma concentrations (Ctrough) of rivaroxaban, coagulation indicators at the Ctrough including activated partial thromboplastin time (APTT) and prothrombin time (PT), and clinical outcomes.
Results
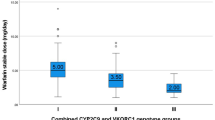
Patients with mutant genotype CYP3A4 (rs2242480, rs2246709, and rs3735451) and CYP3A5 (rs776746) had higher levels of rivaroxaban Ctrough, PT values than that of wild-type. Furthermore, a positive relationship was revealed between Ctrough and PT (r = 0.212, p = 0.007), while no significant correlation was found between Ctrough and APTT. Regarding the clinical outcomes, the minor allele carriers on rs3735451 and the minor allele (A) carriers on rs2246709 were associated with higher incidence of minor bleeding (p = 0.028 and p = 0.038, respectively) and were identified as the independent risk factors of minor bleeding treated with rivaroxaban (p = 0.024 and p = 0.036, respectively), with the receiver operating characteristic (ROC) curve validated (AUC = 0.8956, 95% CI: 0.829–0.962).
Conclusion
The CYP3A4 polymorphisms (rs2242480, rs2246709, and rs3735451) and CYP3A5 rs776746 were associated with variations in rivaroxaban PK/PD. The minor allele (C) carriers on rs3735451 and the minor allele (A) carriers on rs2246709 were correlated with clinical outcomes.



Similar content being viewed by others
Data Availability
The data underlying this study will be available based on reasonable request to the corresponding authors.
Code Availability
The analytic code will be available upon request pending application and approval.
References
Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–76.
Sennesael A-L, Larock A-S, Douxfils J, et al. Rivaroxaban plasma levels in patients admitted for bleeding events: insights from a prospective study. Thrombosis J. 2018;16:28.
Chan KE, Edelman ER, Wenger JB, et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–9.
Coleman CI, Thompson S, Ashton V, et al. Rivaroxaban versus warfarin in African American patients with nonvalvular atrial fibrillation. J Natl Med Assoc. 2020;112(4):395–401.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Wang Y, Chen M, Chen H, et al. Influence of ABCB1 gene polymorphism on rivaroxaban blood concentration and hemorrhagic events in patients with atrial fibrillation. Front Pharmacol. 2021;12:639854.
Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455–66.
Sychev DA, Vardanyan A, Rozhkov A, et al. CYP3A activity and rivaroxaban serum concentrations in russian patients with deep vein thrombosis. Genet Test Mol Biomarkers. 2018;22(1):51–4.
Dong Y, Xu Q, Li R, et al. CYP3A7, CYP3A4, and CYP3A5 genetic polymorphisms in recipients rather than donors influence tacrolimus concentrations in the early stages after liver transplantation. Gene. 2022;809:146007.
Lolita L, Zheng M, Zhang X, et al. The genetic polymorphism of CYP3A4 rs2242480 is associated with sirolimus trough concentrations among adult renal transplant recipients. Curr Drug Metab. 2020;21(13):1052–9.
Wang L, Zeng G, Li J, et al. Association of polymorphism of CYP3A4, ABCB1, ABCC2, ABCG2, NFKB1, POR, and PXR with the concentration of cyclosporin A in allogeneic haematopoietic stem cell transplantation recipients. Xenobiotica. 2021;51(7):852–8.
Gao N, Tang H, Gao L, et al. CYP3A4 and CYP11A1 variants are risk factors for ischemic stroke: a case control study. BMC Neurol. 2020;20(1):77.
Miklic M, Mavri A, Vene N, et al. Intra- and inter- individual rivaroxaban concentrations and potential bleeding risk in patients with atrial fibrillation. Eur J Clin Pharmacol. 2019;75(8):1069–75.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-+.
Spyropoulos AC, Brohi K, Caprini J, et al. Scientific and Standardization Committee Communication: guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: recommendations for standardized reporting of procedural/surgical bleed risk and patient-specific thromboembolic risk. J Thromb Haemost. 2019;17(11):1966–72.
Nakagawa J, Kinjo T, Iizuka M, et al. Impact of gene polymorphisms in drug-metabolizing enzymes and transporters on trough concentrations of rivaroxaban in patients with atrial fibrillation. Basic Clin Pharmacol Toxicol. 2021;128(2):297–304.
Al-Aieshy F, Malmstrom RE, Antovic J, et al. Clinical evaluation of laboratory methods to monitor exposure of rivaroxaban at trough and peak in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72(6):671–9.
Seiffge DJ, Traenka C, Polymeris A, et al. Feasibility of rapid measurement of rivaroxaban plasma levels in patients with acute stroke. J Thromb Thrombolysis. 2017;43(1):112–6.
Gouin-Thibault I, Delavenne X, Blanchard A, et al. Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost. 2017;15(2):273–83.
Zhang P, Wang M, Liu W, et al. Comparison of co-administration of amiodarone and rivaroxaban to co-administration of dronedarone and rivaroxaban for hemorrhage risks after atrial fibrillation ablation. J Interv Card Electrophysiol. 2022;64(1):121–7.
Gosselin RC, Adcock D, Hawes EM, et al. Evaluating the use of commercial drug-specific calibrators for determining PT and APTT reagent sensitivity to dabigatran and rivaroxaban. Thromb Haemost. 2015;113(1):77–84.
Kreutz R, Persson PB, Kubitza D, et al. Dissociation between the pharmacokinetics and pharmacodynamics of once-daily rivaroxaban and twice-daily apixaban: a randomized crossover study. J Thromb Haemost. 2017;15(10):2017–28.
Zhang Y, Qian Q, Qian G, et al. Laboratory monitoring of rivaroxaban and assessment of its bleeding risk. Br J Biomed Sci. 2016;73(3):134–9.
Douxfils J, Mullier F, Loosen C, et al. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130(6):956–66.
Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti-Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol. 2016;38(5):505–13.
Baglin T, Hillarp A, Tripodi A, et al. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2013;1(4):756–60.
Potpara TS, Lip GYH. Oral anticoagulant therapy in atrial fibrillation patients at high stroke and bleeding risk. Prog Cardiovasc Dis. 2015;58(2):177–94.
Mitrovic D, Folkeringa R, Veeger N, et al. Minor bleeding in patients with atrial fibrillation using a non-vitamin-K antagonist oral anticoagulant. Curr Med Res Opin. 2020;36(10):1571–6.
Author information
Authors and Affiliations
Contributions
Xiaoye Li, Zhichun Gu, Chunlai, and Qianzhou Lv contributed to the study conception and design. Zi Wang and Qing Xu contributed to the acquisition of data. Xiaoye Li and Zhichun Gu contributed to the analysis and interpretation of data. Xiaoye Li and Qianzhou Lv contributed to the drafting of the manuscript. Chunlai Ma and Qianzhou Lv contributed to critical revision. All of the authors agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. The Ethics Committee of Zhongshan Hospital, Fudan University, approved this study.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All authors have given their consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Gu, Z., Wang, Z. et al. Mutant CYP3A4/5 Correlated with Clinical Outcomes by Affecting Rivaroxaban Pharmacokinetics and Pharmacodynamics in Patients with Atrial Fibrillation. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07495-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07495-4