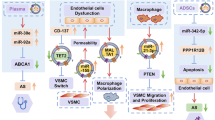
Abstract
Purpose
Delayed re-endothelialization after coronary drug-eluting stent implantation is associated with an increased incidence of late in-stent thrombosis. Serum exosomes exhibit controversial effects on promoting endothelialization. This study aimed to compare the angiogenic effects of serum exosomes derived from patients with acute myocardial infarction (AMI) and AMI plus diabetes mellitus (DM) and to explore the underlying mechanisms.
Methods
Serum exosomes derived from patients in the control (Con-Exos), AMI (AMI-Exos), and AMI plus DM (AMI+DM-Exos) groups were isolated and identified using standard assays. CCK-8, wound healing, and tube formation assays were performed to detect the angiogenic abilities of serum exosomes on rapamycin-conditioned human umbilical vein endothelial cells (HUVECs). Differential proteomic profiles between AMI-Exos and AMI+DM-Exos were analyzed by mass spectrometry. The effects and potential mechanisms of exosomal angiopoietin-like 6 (ANGPTL6) were investigated.
Results
Functional assays indicated that compared with Con-Exos, AMI-Exos enhanced, whereas AMI+DM-Exos inhibited the cell proliferation, migration, and tube formation of rapamycin-conditioned HUVECs. Subsequently, 28 differentially expressed proteins between AMI-Exos and AMI+DM-Exos were identified, which were correlated with material transportation, immunity, and inflammatory reaction. Moreover, ANGPTL6 was highly enriched in AMI-Exos. Overexpression and knockdown of ANGPTL6 enhanced and inhibited angiogenesis, respectively. Furthermore, the effect of ANGPTL6 on angiogenesis was mediated via the activation of ERK 1/2, JNK, and p38 pathways. The inhibition of ERK 1/2 signaling markedly attenuated the migration abilities of overexpressing ANGPTL6.
Conclusion
Diabetes impairs the regenerative capacities of serum exosomes. Exosomal ANGPTL6 contributes to endothelial repair and is a novel therapeutic target for enhanced stent endothelization.
Graphical abstract







Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
References
Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–41.
Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Liu S, Zhi J, Li S, et al. Progress on precise regulation of vascular intimal repair by surface coating of vascular stent. Curr Drug Deliv. 2021;18(7):862–73.
Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9(8):439–53.
Krishnagopal A, Reddy A, Sen D. Stent-mediated gene and drug delivery for cardiovascular disease and cancer: a brief insight. J Gene Med. 2017;19(5):e2954.
He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11(6):e468.
Geng T, Song ZY, Xing JX, Wang BX, Dai SP, Xu ZS. Exosome derived from coronary serum of patients with myocardial infarction promotes angiogenesis through the miRNA-143/IGF-IR pathway. Int J Nanomedicine. 2020;15:2647–58.
Gao L, Mei S, Zhang S, et al. Cardio-renal exosomes in myocardial infarction serum regulate proangiogenic paracrine signaling in adipose mesenchymal stem cells. Theranostics. 2020;10(3):1060–73.
Davidson SM, Riquelme JA, Takov K, et al. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J Cell Mol Med. 2018;22(1):141–51.
Liu Y, Xu J, Gu R, et al. Circulating exosomal miR-144-3p inhibits the mobilization of endothelial progenitor cells post myocardial infarction via regulating the MMP9 pathway. Aging (Albany NY). 2020;12(16):16294–303.
Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
Wang W, Zhao Y, Li H, et al. Exosomes secreted from mesenchymal stem cells mediate the regeneration of endothelial cells treated with rapamycin by delivering pro-angiogenic microRNAs. Exp Cell Res. 2021;399(1):112449.
Gao F, Jiao F, Xia C, et al. A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO2. Chem Sci. 2018;10(6):1579–88.
He R, Zhao Z, Yang Y, Liang X. Using bioinformatics and metabolomics to identify altered granulosa cells in patients with diminished ovarian reserve. PeerJ. 2020;8:e9812.
Urano T, Ito Y, Akao M, et al. Angiopoietin-related growth factor enhances blood flow via activation of the ERK1/2-eNOS-NO pathway in a mouse hind-limb ischemia model. Arterioscler Thromb Vasc Biol. 2008;28(5):827–34.
He L, Huang X, Kanisicak O, et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest. 2017;127(8):2968–81.
Li Z, Solomonidis EG, Meloni M, et al. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur Heart J. 2019;40(30):2507–20.
Hu S, Li Z, Shen D, et al. Exosome-eluting stents for vascular healing after ischaemic injury. Nat Biomed Eng. 2021;5(10):1174–88.
Arnold SV, Lipska KJ, Li Y, et al. Prevalence of glucose abnormalities among patients presenting with an acute myocardial infarction. Am Heart J. 2014;168(4):466–70.
Li W, Li C, Zhou T, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16(1):145.
Pardo F, Villalobos-Labra R, Sobrevia B, Toledo F, Sobrevia L. Extracellular vesicles in obesity and diabetes mellitus. Mol Asp Med. 2018;60:81–91.
Li H, Liao Y, Gao L, et al. Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the miR-939-mediated nitric oxide signaling pathway. Theranostics. 2018;8(8):2079–93.
Xiong Y, Chen L, Yan C, et al. Circulating exosomal miR-20b-5p inhibition restores wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 2020;16(3):e1904044.
Carbone C, Piro G, Merz V, et al. Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci. 2018;19(2):431.
Oike Y, Yasunaga K, Ito Y, et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc Natl Acad Sci U S A. 2003;100(16):9494–9.
Chen E, Tang C, Peng K, Cheng X, Wei Y, Liu T. ANGPTL6-mediated angiogenesis promotes alpha fetoprotein-producing gastric cancer progression. Pathol Res Pract. 2019;215(8):152454.
Oike Y, Ito Y, Maekawa H, et al. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood. 2004;103(10):3760–5.
Zhang Y, Hu X, Tian R, et al. Angiopoietin-related growth factor (AGF) supports adhesion, spreading, and migration of keratinocytes, fibroblasts, and endothelial cells through interaction with RGD-binding integrins. Biochem Biophys Res Commun. 2006;347(1):100–8.
Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89(6):867–82.
Yuan X, Han L, Fu P, et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab Investig. 2018;98(6):783–98.
He M, Li L, Wei X, et al. Xiaoyao powder improves endometrial receptivity via VEGFR-2-mediated angiogenesis through the activation of the JNK and P38 signaling pathways. J Ethnopharmacol. 2022;282:114580.
Zhang Q, Wang L, Wang S, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7(1):78.
Toral M, Jimenez R, Montoro-Molina S, et al. Thyroid hormones stimulate L-arginine transport in human endothelial cells. J Endocrinol. 2018;239(1):49–62.
Moon C, Han JR, Park HJ, Hah JS, Kang JL. Synthetic RGDS peptide attenuates lipopolysaccharide-induced pulmonary inflammation by inhibiting integrin signaled MAP kinase pathways. Respir Res. 2009;10(1):18.
Kang SG, Yi HS, Choi MJ, et al. ANGPTL6 expression is coupled with mitochondrial OXPHOS function to regulate adipose FGF21. J Endocrinol. 2017;233(1):105–18.
Takov K, Yellon DM, Davidson SM. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J Extracell Vesicles. 2017;6(1):1388731.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 81970281), Academic Promotion Programme of Shandong First Medical University (Grant number 2019QL012), Natural Science Foundation of Shandong Province (Grant number ZR2021QH130), and Projects of Medical and Health Technology Development Program in Shandong province (Grant number 2017WS087).
Author information
Authors and Affiliations
Contributions
Weizong Wang, Jiangrong Wang, and Yinglong Hou designed the study. Weizong Wang, Yixin Zhao, and Pengju Zhu performed the experiments, collected, and assembled data. Weizong Wang wrote the manuscript. Xiaomeng Jia analyzed and interpreted the data. Cong Wang prepared all the figures. Qingbin Zhang participated in editing the manuscript. Hao Li provided technical support. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study design was approved by The Ethics Committee of The First Affiliated Hospital of Shandong First Medical University. The procedures used in this study were in accordance with the tenets of the 1964 Helsinki Declaration.
Consent to Participate
Written informed consent was acquired from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Supplementary Fig. 1
Transfection efficiency of ANGPTL6 overexpression plasmid in HUVECs treated with rapamycin. **P < 0.001 (PNG 130 kb)

Supplementary Fig. 2
Comparison of different ANGPTL6 siRNAs for transfection efficiency in HUVECs treated with rapamycin. **P < 0.001. CON = scramble control (PNG 111 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Zhao, Y., Zhu, P. et al. Differential Proteomic Profiles of Coronary Serum Exosomes in Acute Myocardial Infarction Patients with or Without Diabetes Mellitus: ANGPTL6 Accelerates Regeneration of Endothelial Cells Treated with Rapamycin via MAPK Pathways. Cardiovasc Drugs Ther 38, 13–29 (2024). https://doi.org/10.1007/s10557-022-07365-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07365-5