Abstract
Purpose
Endothelial progenitor cells (EPCs) play a critical role in repairing damaged vessels and triggering ischemic angiogenesis, but their number is reduced and function is impaired under diabetic conditions. Improving EPC function has been considered a promising strategy to ameliorate diabetic vascular complications. In the present study, we aim to investigate whether and how CXCR7 agonist TC14012 promotes the angiogenic function of diabetic EPCs.
Methods
High glucose (HG) treatment was used to mimic the hyperglycemia in diabetes. Tube formation, cell scratch recovery and transwell assay, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, and cleaved-caspase3 expression were used to evaluate the angiogenic capability, cell migration, and apoptosis of EPCs, respectively. Hind limb ischemia (HLI) model was used to appraise the ability of TC14012 in promoting diabetic ischemic angiogenesis in vivo.
Results
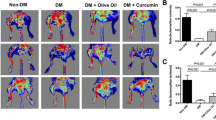
HG treatment impaired EPC tube formation and migration, and induced EPC apoptosis and oxidative damage, while TC14012 rescued tube formation and migration, and prevented HG-induced apoptosis and oxidative damage of EPCs. Furthermore, these beneficial effects of TC14012 on EPCs were attenuated by specific siRNAs against CXCR7, validating that CXCR7 is a functional target of TC14012 in EPCs. Mechanistic studies demonstrated that HG treatment reduced CXCR7 expression in EPCs, and impaired Akt and endothelial nitric oxide synthase (eNOS) phosphorylation and nitric oxide (NO) production; similarly, these signal impairments in HG-exposed EPCs could be rescued by TC14012. However, the protective effects of TC14012 on tube formation and migration, Akt and eNOS phosphorylation, and NO production in HG-treated EPCs were almost completely abolished by siRNAs against CXCR7 or Akt specific inhibitor wortmannin. More importantly, in vivo study showed that TC14012 administration enhanced blood perfusion recovery and angiogenesis in the ischemic hind limb and increased the EPC number in peripheral circulation of db/db mice, demonstrating the capability of TC14012 in promoting EPC mobilization and ischemia angiogenic function.
Conclusion
TC14012 can prevent EPCs from HG-induced dysfunction and apoptosis, improve eNOS activity and NO production via CXCR7/Akt signal pathway, and promote EPC mobilization and diabetic ischemia angiogenesis.








Similar content being viewed by others
Data Availability
All data relevant to the study are included in the article or uploaded as supplementary information.
Code Availability
Not applicable.
References
Rodriguez-Gutierrez R, Gonzalez-Gonzalez J, Zuñiga-Hernandez J, et al. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ (Clin Res ed). 2019;367: l5887.
Newman JD, Vani AK, Aleman JO, et al. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1856–69.
Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;58(10085):63.
Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9(5):434–49.
Wasserman DH, Wang TJ, Brown NJ. The vasculature in prediabetes. Circ Res. 2018;122(8):1135–50.
Chopra H, Hung MK, Kwong DL, et al. Insights into endothelial progenitor cells: origin, classification, potentials, and prospects. Stem Cells Int. 2018;2018:9847015.
Aicher A, Heeschen C, Sasaki K-I, et al. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114(25):2823–30.
Yan X, Dai X, He L, et al. A novel CXCR4 antagonist enhances angiogenesis via modifying the ischaemic tissue environment. J Cell Mol Med. 2017;21(10):2298–307.
Dai X, Yan X, Zeng J, et al. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3β/Fyn-mediated Nrf2 activation in diabetic limb ischemia. Circ Res. 2017;120(5):e7–23.
Williamson K, Stringer SE, Alexander MY. Endothelial progenitor cells enter the aging arena. Front Physiol. 2012;3:30.
Geng J, Wang L, Qu M, et al. Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1alpha. Stem Cell Res Ther. 2017;8(1):163.
Grochot-Przeczek A, Kotlinowski J, Kozakowska M, et al. Heme oxygenase-1 is required for angiogenic function of bone marrow-derived progenitor cells: role in therapeutic revascularization. Antioxid Redox Signal. 2014;20(11):1677–92.
Xing Z, Zhao C, Liu H, et al. Endothelial progenitor cell-derived extracellular vesicles: a novel candidate for regenerative medicine and disease treatment. Adv Healthc Mater. 2020;9(12): e2000255.
Wang K, Dai X, He J, et al. Endothelial overexpression of metallothionein prevents diabetes-induced impairment in ischemia angiogenesis through preservation of HIF-1alpha/SDF-1/VEGF signaling in endothelial progenitor cells. Diabetes. 2020;69(8):1779–92.
Dai X, Yan X, Zeng J, et al. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3beta/Fyn-mediated Nrf2 activation in diabetic limb ischemia. Circ Res. 2017;120(5):e7–23.
Cun Y, Diao B, Zhang Z, et al. Role of the stromal cell derived factor-1 in the biological functions of endothelial progenitor cells and its underlying mechanisms. Exp Ther Med. 2021;21(1):39.
Dai X, Tan Y, Cai S, et al. The role of CXCR7 on the adhesion, proliferation and angiogenesis of endothelial progenitor cells. J Cell Mol Med. 2011;15(6):1299–309.
Jujo K, Hamada H, Iwakura A, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107(24):11008–13.
Yan X, Cai S, Xiong X, et al. Chemokine receptor CXCR7 mediates human endothelial progenitor cells survival, angiogenesis, but not proliferation. J Cell Biochem. 2012;113(4):1437–46.
Gravel S, Malouf C, Boulais PE, et al. The peptidomimetic CXCR4 antagonist TC14012 recruits beta-arrestin to CXCR7: roles of receptor domains. J Biol Chem. 2010;285(49):37939–43.
Ishizuka M, Harada M, Nomura S, et al. CXCR7 ameliorates myocardial infarction as a beta-arrestin-biased receptor. Sci Rep. 2021;11(1):3426.
Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505(7481):97–102.
Zhang M, Qiu L, Zhang Y, et al. CXCL12 enhances angiogenesis through CXCR7 activation in human umbilical vein endothelial cells. Sci Rep. 2017;7(1):8289.
Zhang S, Yue J, Ge Z, et al. Activation of CXCR7 alleviates cardiac insufficiency after myocardial infarction by promoting angiogenesis and reducing apoptosis. Biomed Pharmacother. 2020;127: 110168.
Cao Z, Lis R, Ginsberg M, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22(2):154–62.
Fan X, He L, Dai Q, et al. Interleukin-1beta augments the angiogenesis of endothelial progenitor cells in an NF-kappaB/CXCR7-dependent manner. J Cell Mol Med. 2020;24(10):5605–14.
Hao H, Hu S, Chen H, et al. Loss of endothelial CXCR7 impairs vascular homeostasis and cardiac remodeling after myocardial infarction: implications for cardiovascular drug discovery. Circulation. 2017;135(13):1253–64.
Juarez JG, Harun N, Thien M, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119(3):707–16.
Zhang Z, Wang S, Zhou S, et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. 2014;77:42–52.
Huang CC, Kuo HM, Wu PC, et al. Soluble delta-like 1 homolog (DLK1) stimulates angiogenesis through Notch1/Akt/eNOS signaling in endothelial cells. Angiogenesis. 2018;21(2):299–312.
Icli B, Wu W, Ozdemir D, et al. MicroRNA-615-5p regulates angiogenesis and tissue repair by targeting AKT/eNOS (protein kinase B/endothelial nitric oxide synthase) signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2019;39(7):1458–74.
Boopathy GTK, Kulkarni M, Ho SY, et al. Cavin-2 regulates the activity and stability of endothelial nitric-oxide synthase (eNOS) in angiogenesis. J Biol Chem. 2017;292(43):17760–76.
Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–5.
Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27(2):263–5.
Ishizuka M, Harada M, Nomura S, et al. CXCR7 ameliorates myocardial infarction as a β-arrestin-biased receptor. Sci Rep. 2021;11(1):3426.
Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–10.
Tan Y, Li Y, Xiao J, et al. A novel CXCR4 antagonist derived from human SDF-1beta enhances angiogenesis in ischaemic mice. Cardiovasc Res. 2009;82(3):513–21.
Jiao C, Fricker S, Schatteman GC. The chemokine (C-X-C motif) receptor 4 inhibitor AMD3100 accelerates blood flow restoration in diabetic mice. Diabetologia. 2006;49(11):2786–9.
Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116(2):163–73.
Hamed S, Brenner B, Roguin A. Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2. Cardiovasc Res. 2011;91(1):9–15.
Ambasta RK, Kohli H, Kumar P. Multiple therapeutic effect of endothelial progenitor cell regulated by drugs in diabetes and diabetes related disorder. J Transl Med. 2017;15(1):185.
Albiero M, Poncina N, Ciciliot S, et al. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes. 2015;64(8):2957–68.
Zhang J, Zhang Y, Xin S, et al. CXCR7 suppression modulates macrophage phenotype and function to ameliorate post-myocardial infarction injury. Inflammation Res. 2020;69(5):523–32.
Funding
This study was supported by a Junior Faculty Award (1–13-JF-53) from the American Diabetes Association, National Key Research and Development Program of China (2017YFA0506000), National Natural Science Foundation of China (81770305, 81873466, 82070834), Natural Science Foundation of Zhejiang Province (LY22H070005, LY22H020005), Foundation for Distinguished Young Scholars of Sichuan Province (2019JDJQ0042), Key Research and Development Support Plan of Chengdu (2019-YF05-00275-SN), Fund of Development and Regeneration Key Laboratory of Sichuan Province (SYS18-04), Grant from Science and Technology Bureau of Wenzhou (Y20210010), and Basic Scientific Research Foundation of Wenzhou Medical University (KYYW201907).
Author information
Authors and Affiliations
Contributions
Kai Wang, Xiaoqing Yan, and Yi Tan conceived and designed the study. Kai Wang, Shiyue Sun, Guigui Zhang, Zixian Lu, Hui Chen, Xia Fan, Chunjie Gu, Xiaohong Pan, Oscar Chen, Qian Lin, Xiao Wang, and Chaosheng Lu performed in vitro cell culture, cell biology, molecular biology experiments, and histology analysis. Kai Wang and Xiaozhen Dai performed in vivo therapeutic studies in mice. Kai Wang, Xiaoqing Yan, and Yi Tan wrote the manuscript. Kai Wang and Lu Cai edited the manuscript with important intellectual content. Xiaoqing Yan and Yi Tan supervised this study.
Corresponding authors
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Declaration of Helsinki. All protocols were approved by the Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University.
Consent to Participate
All samples were collected with written informed consent of the participants.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10557_2022_7337_MOESM1_ESM.pdf
Fig. S1. TC14012 protects EPCs from HG-induced impairment of migration. EPCs were exposed to high glucose (HG, 33 mmol/L) with or without TC14012 (TC, 5 μmol/L) treatment for 24 h, the equivalent concentration of mannitol (Man) was used as osmotic control. The migration of EPCs was determined by transwell assay (A) and the migrated cells were counted using ImageJ (B). Three independent experiments were performed. Data were expressed as Mean ± SD. *P<0.05 vs. Man; #P< 0.05 vs. HG. Fig. S2. TC14012 decreased MDA content of HG-treated EPCs. The MDA content in EPCs was detected using MDA detecting kit. Three independent experiments were performed. Data were normalized to cell number and expressed as Mean ± SD. *P<0.05 vs. Man; #P< 0.05 vs. HG. Fig. S3. CXCR7 knockdown abolishes the protective effect of TC14012 on migration of HG-treated EPCs. Three siRNAs against CXCR7 sequences were chosen and RT-PCR was used to detect knockdown efficiency (A). CXCR7-siRNA-441 with the highest transfection efficiency was selected, CXCR7 protein expression was evaluated by Western blot (B). The migration of EPCs was determined by transwell assay (C) and the migrated cells were counted using ImageJ (D). Three independent experiments were performed. Data were expressed as Mean ± SD. *P<0.05 vs. Ctrl-siRNA-Man; #P< 0.05 vs. HG; & P<0.05 vs. CXCR7-siRNA-Man. Fig. S4. Akt inhibitor wortmannin abolisheS the protective effect of TC14012 on migration of HG-treated EPCs. EPCs were exposed to high glucose (HG) with or without TC14012 (TC) treatment in the present of absent of wortmannin (Wort) for 24 h, the equivalent concentration of mannitol (Man) was used as osmotic control. The migration of EPCs was determined by transwell assay (A) and the migrated cells were counted using ImageJ (B). Three independent experiments were performed. Data were expressed as Mean ± SD. *P<0.05 vs. Man; #P< 0.05 vs. HG. Fig. S5. TC14012 did not affect blood glucose of db/db mice. Random blood glucose of mice was tested using glucose testing strips (Roche). n=6, 7 per group, respectively. (PDF 299 KB)
Rights and permissions
About this article
Cite this article
Wang, K., Sun, S., Zhang, G. et al. CXCR7 Agonist TC14012 Improves Angiogenic Function of Endothelial Progenitor Cells via Activating Akt/eNOS Pathway and Promotes Ischemic Angiogenesis in Diabetic Limb Ischemia. Cardiovasc Drugs Ther 37, 849–863 (2023). https://doi.org/10.1007/s10557-022-07337-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07337-9