Abstract
Purpose
The CD36 scavenger receptor is a mediator of both atherogenesis and thrombosis. We aimed to investigate the prognostic value of CD36+ microparticles (MPs) released from platelets for cardiovascular event presentation in coronary artery disease (CAD) patients and the effects of different antiplatelet drugs on MPs.
Methods
A total of 101 aspirin-treated CAD patients, who were planned to undergo coronary angiography (CAG), were randomized to either a standard clopidogrel regimen or ticagrelor treatment. Total Annexin V-(AV)+ MPs, CD61+/AV+ MPs, and CD36+/CD61+/AV+ MPs were quantified by flow cytometry at baseline, before and immediately after the operation. The ADP-induced platelet inhibition rate was measured by thromboelastogram (TEG) examination 1 h before the operation.
Results
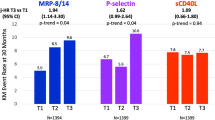
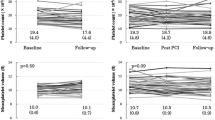
The baseline levels of CD36+/CD61+/AV+ MPs were significantly increased in percutaneous coronary intervention (PCI) patients (n = 52) compared to no-PCI patients (n = 49) (p < 0.05). A ROC-curve clustered model for CD36+/CD61+/AV+ MPs at baseline predicted an increased risk of PCI [p = 0.009, AUC = 0.761 (95%CI: 0.601 to 0.922)]. Moreover, TEG examination showed that the preoperative proportion of CD36+/CD61+/AV+ MPs was significantly negatively correlated with R time and K time (r = − 0.236, p = 00.026; r = − 0.288, p = 0.006), and positively correlated with MAADP (r = 0.226, p = 0.045). Subgroup analysis of PCI group showed that the platelet inhibition rate of ticagrelor was significantly higher (66.05% ± 28.76% vs.31.01% ± 27.33%, p < 0.001), and the number of AV+ MPs, CD61+/AV+ MPs, and CD36+/CD61+/AV+ MPs before the operation was significantly lower than clopidogrel (p < 0.05, all).
Conclusion
The high levels of CD36+ MPs derived from activated platelets are related to an increased risk of PCI in CAD patients. Ticagrelor significantly reduced the number of CD61+/AV+ MPs and CD36+/CD61+/AV+ MPs.
This trial registration number is ChiCTR1800014908 and the date of registration is 2018.05.01.


Similar content being viewed by others
Data Availability
Availability of data and material has been described in the manuscript. They are freely available to any scientist who wishes to use them without breaching participant confidentiality.
References
Sluijter J, Davidson S, Boulanger C, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2018;114:19–34.
Shantsila E, Kamphuisen P, Lip G. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemostasis. 2010;8:2358–68.
Voukalis C, Shantsila E, Lip G. Microparticles and cardiovascular diseases. Ann Med. 2019;51:193–223.
Sinauridze E, Kireev D, Popenko N, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34.
Tan K, Tayebjee M, Lynd C, Blann A, Lip G. Platelet microparticles and soluble P selectin in peripheral artery disease: relationship to extent of disease and platelet activation markers. Ann Med. 2005;37:61–6.
Williams M, Rogers H, Wang N, Ziegelstein R. Do platelet-derived microparticles play a role in depression, inflammation, and acute coronary syndrome? Psychosomatics. 2014;55:252–60.
Biasucci L, Porto I, Di Vito L, et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ J. 2012;76:2174–82.
Chen Y, Chen C, Wallace C, et al. Levels of circulating microparticles in patients with chronic cardiorenal disease. J Atheroscler Thromb. 2015;22:247–56.
Chen Y, Xiao Y, Lin Z, Xiao X, He C, Bihl JC, et al. The role of circulating platelets microparticles and platelet parameters in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2015;24:2313–20.
Chiva-Blanch G, Suades R, Crespo J, Peña E, Padró T, Jiménez-Xarrié E, et al. Microparticle shedding from neural progenitor cells and vascular compartment cells is increased in ischemic stroke. PLoS One. 2016;11:e0148176.
Yang Y, Luo H, Liu S, et al. Platelet microparticles-containing miR-4306 inhibits human monocyte-derived macrophages migration through VEGFA/ERK1/2/NF-κB signaling pathways. Clin Exp Hypertens (New York, NY : 1993). 2019;41:481–91.
Hartopo AB, Puspitawati I, Gharini PP, Setianto BY. Platelet microparticle number is associated with the extent of myocardial damage in acute myocardial infarction. Arch Med Sci. 2016;12:529–37.
Zhou B, Li J, Chen S, et al. Time course of various cell origin circulating microparticles in ST-segment elevation myocardial infarction patients undergoing percutaneous transluminal coronary intervention. Exp Ther Med. 2016;11:1481–6.
Chen K, Febbraio M, Li W, Silverstein R. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102:1512–9.
Yang M, Silverstein R. CD36 signaling in vascular redox stress. Free Radic Biol Med. 2019;136:159–71.
Silverstein R. Inflammation, atherosclerosis, and arterial thrombosis: role of the scavenger receptor CD36. Cleve Clin J Med. 2009;76:S27–30.
Ghosh A, Li W, Febbraio M, Espinola RG, McCrae K, Cockrell E, et al. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest. 2008;118:1934–43.
Loudová M, Krejsek J. Platelets activation in patients undergoing PTCA and their responsiveness after in vitro stimulation. Acta Med (Hradec Kralove). 2003;46:183–8.
Wilhelmsen P, Kjær J, Thomsen K, et al. Elevated platelet expression of CD36 may contribute to increased risk of thrombo-embolism in active inflammatory bowel disease. Arch Physiol Biochem. 2013;119:202–8.
Parker W, Storey R. Ticagrelor: agonising over its mechanisms of action. Blood. 2016;128:2595–7.
Aungraheeta R, Conibear A, Butler M, Kelly E, Nylander S, Mumford A, et al. Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood. 2016;128:2717–28.
Rabani V, Montange D, Meneveau N, Davani S. Impact of ticagrelor on P2Y1 and P2Y12 localization and on cholesterol levels in platelet plasma membrane. Platelets. 2018;29:709–15.
França C, Pinheiro L, Izar M, et al. Endothelial progenitor cell mobilization and platelet microparticle release are influenced by clopidogrel plasma levels in stable coronary artery disease. Circ J. 2012;76:729–36.
Dasgupta S, Le A, Chavakis T, Rumbaut R, Thiagarajan P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012;125:1664–72.
Gensini G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606.
Théry C, Witwer K, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracellular vesicles. 2018;7:1535750.
R. Lacroix, C. Judicone, M. Mooberry, M. Boucekine, N. S. Key, F. Dignat-George, (2013) Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. Journal of Thrombosis and Haemostasis 11 (6):1190-1193
Ferroni P, Riondino S, Vazzana N, Santoro N, Guadagni F, Davì G. Biomarkers of platelet activation in acute coronary syndromes. Thromb Haemost. 2012;108:1109–23.
Trappenburg M, van Schilfgaarde M, Marchetti M, et al. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94:911–8.
Alkhatatbeh M, Mhaidat N, Enjeti A, Lincz L, Thorne R. The putative diabetic plasma marker, soluble CD36, is non-cleaved, non-soluble and entirely associated with microparticles. J Thromb Haemostasis. 2011;9:844–51.
Lee B, Lin K, Hu C, Lo S. Thromboelastography characterized CD36 null subjects as slow clot formation and indicative of hypocoagulability. Thromb Res. 2019;178:79–84.
Döhrmann M, Makhoul S, Gross K, Krause M, Pillitteri D, Auer C, et al. CD36-fibrin interaction propagates FXI-dependent thrombin generation of human platelets. FASEB J. 2020;34:9337–57.
Signorelli S, Oliveri Conti G, Fiore M, et al. Platelet-derived microparticles (MPs) and thrombin generation velocity in deep vein thrombosis (DVT): results of a case-control study. Vasc Health Risk Manag. 2020;16:489–95.
Sun C, Zhao W, Chen Y, Hu H. Higher plasma concentrations of platelet microparticles in patients with acute coronary syndrome: a systematic review and meta-analysis. Can J Cardiol. 2016;32:1325.e1321–10.
Suades R, Padró T, Crespo J, Ramaiola I, Martin-Yuste V, Sabaté M, et al. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int J Cardiol. 2016;202:378–87.
Botha J, Velling Magnussen L, Nielsen M, et al. Microvesicles correlated with components of metabolic syndrome in men with type 2 diabetes mellitus and lowered testosterone levels but were unaltered by testosterone therapy. J Diabetes Res. 2017;2017:4257875.
Chiva-Blanch G, Crespo J, Suades R, Arderiu G, Padro T, Vilahur G, et al. CD142+/CD61+, CD146+ and CD45+ microparticles predict cardiovascular events in high risk patients following a Mediterranean diet supplemented with nuts. Thromb Haemost. 2016;116:103–14.
Heemskerk J, Bevers E, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–93.
Biró E, Akkerman J, Hoek F, et al. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J Thromb Haemostasis. 2005;3:2754–63.
Qi Z, Hu L, Zhang J, et al. PCSK9 enhances platelet activation, thrombosis, and myocardial infarct expansion by binding to platelet CD36. Circulation. 2020.
Acknowledgements
We thank all study subjects and investigators for their participation in this project.
Code Availability
All statistical analyses were generated with Statistical Package for Social Sciences (IBM SPSS version 22.0, Armonk, New York, USA).
Funding
This study was supported by the National Natural Science Foundation of China Youth Program (Grant No. 81900314), the Tianjin Science and Technology Committee Foundation (Grant No. 17JCYBJC27800), the Tianjin Health and Family Planning Commission Foundation (Grant No. 16KG120), and the Tianjin Research Innovation Project for Postgraduate Students (Grant No.2019YJS181). Additional funding was provided by the Key Laboratory Fund of the Second Hospital of Tianjin Medical University (Grant No.2018ZDSYS10) and the Science & Technology Development Fund of Tianjin Education Commission for Higher Education (Grant No.2017KJ205).
Author information
Authors and Affiliations
Contributions
Xue Zhou and Xing Liu performed the experiments, assisted in data collection and analysis, and writing. Xue Zhou and Xing Liu contributed equally to this work. Hongmei Liu, Shuang Dou, Kangyin Chen, Xiaowei Zhang, Weiding Wang, and Xuewen Wang collected blood samples. Jingjin Che contributed to the design, interpretation of results, and final approval.
Corresponding author
Ethics declarations
Ethics Approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
All participants provided written informed consent before enrollment in this study.
Consent for Publication
Written informed consent was obtained from all study participants.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Zhou, X., Liu, X., Liu, H. et al. CD36+/CD61+ Microparticles Correlate with the Risk of Percutaneous Cardiac Interventions in Coronary Artery Disease Patients and the Effects of Ticagrelor. Cardiovasc Drugs Ther 36, 455–465 (2022). https://doi.org/10.1007/s10557-021-07184-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07184-0