Abstract
Purpose
Left ventricular hypertrophy (LVH) is a cardiovascular complication highly prevalent in patients with chronic kidney disease (CKD). Previous studies analyzing 1α-hydroxylase or vitamin D receptor (Vdr) knockout mice revealed active vitamin D as a promising agent inhibiting LVH progression. Paricalcitol, an active vitamin D analog, failed to suppress the progression of LV mass index (LVMI) in pre-dialysis patients with CKD. As target genes of activated VDR differ depending on its agonists, we examined the effects of maxacalcitol (22-oxacalcitriol: OCT), a less calcemic active vitamin D analog, on LVH in hemodialysis patients and animal LVH models with renal insufficiency.
Methods
In retrospective cohort study, patients treated with OCT who underwent hemodialysis were enrolled. Using cardiac echocardiography, LV mass was evaluated by the area-length method. In animal study, angiotensin II (Ang II)-infused Wister rats with heminephrectomy or Ang II-stimulated neonatal rat ventricular myocytes (NRVM) were treated with OCT.
Results
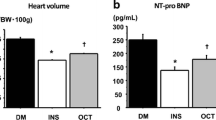
OCT significantly inhibited the progression of LVMI in hemodialysis patients. In Ang II-infused heminephrectomized rats, OCT suppressed the progression of LVH in a blood pressure-independent manner. OCT also suppressed the activity of calcineurin in the left ventricle of model rats. Specifically, OCT reduced the protein levels of calcineurin A, but not the mRNA levels of Ppp3ca (calcineurin Aα). Luciferase assays showed that OCT increased the promoter activity of Fbxo32 (atrogin1), an E3 ubiquitin ligase targeting calcineurin A. Finally, OCT promoted ubiquitination and degradation of calcineurin A.
Conclusion
Our works indicated that OCT retards progression of LVH through calcineurin-NFAT pathway, which reveal a novel aspect of OCT in attenuating pathological LVH.






Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available on request.
References
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
Das M, Aronow WS, McClung JA, Belkin RN. Increased prevalence of coronary artery disease, silent myocardial ischemia, complex ventricular arrhythmias, atrial fibrillation, left ventricular hypertrophy, mitral annular calcium, and aortic valve calcium in patients with chronic renal insufficiency. Cardiol Rev. 2006;14(1):14–7.
Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, et al. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis. 2010;55(1):21–30.
Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46(2):320–7.
Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34(1):125–34.
Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65(4):1492–8.
Untersteller K, Girerd N, Duarte K, Rogacev KS, Seiler-Mussler S, Fliser D, et al. NT-proBNP and echocardiographic parameters for prediction of cardiovascular outcomes in patients with CKD stages G2-G4. Clin J Am Soc Nephrol. 2016;11(11):1978–88.
Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–32.
Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–9.
Dardenne O, Prud'homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142(7):3135–41.
Nakano C, Hamano T, Fujii N, Matsui I, Tomida K, Mikami S, et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7(5):810–9.
Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23(10):1725–34.
Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104(43):16810–5.
Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–84.
Takeyama K, Masuhiro Y, Fuse H, Endoh H, Murayama A, Kitanaka S, et al. Selective interaction of vitamin D receptor with transcriptional coactivators by a vitamin D analog. Mol Cell Biol. 1999;19(2):1049–55.
Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–231.
Inoue K, Matsui I, Hamano T, Fujii N, Shimomura A, Nakano C, et al. Maxacalcitol ameliorates tubulointerstitial fibrosis in obstructed kidneys by recruiting PPM1A/VDR complex to pSmad3. Lab Investig. 2012;92(12):1686–97.
Matsui I, Hamano T, Tomida K, Inoue K, Takabatake Y, Nagasawa Y, et al. Active vitamin D and its analogue, 22-oxacalcitriol, ameliorate puromycin aminonucleoside-induced nephrosis in rats. Nephrol Dial Transplant. 2009;24(8):2354–61.
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–28.
Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, et al. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87(12):E61–8.
Bush E, Fielitz J, Melvin L, Martinez-Arnold M, McKinsey TA, Plichta R, et al. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc Natl Acad Sci U S A. 2004;101(9):2870–5.
Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, et al. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One. 2013;8(7):e68893.
Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122(4):361–9.
Kusunoki Y, Matsui I, Hamano T, Shimomura A, Mori D, Yonemoto S, et al. Excess 25-hydroxyvitamin D3 exacerbates tubulointerstitial injury in mice by modulating macrophage phenotype. Kidney Int. 2015;88(5):1013–29.
Hashimoto N, Matsui I, Ishizuka S, Inoue K, Matsumoto A, Shimada K, et al. Lithocholic acid increases intestinal phosphate and calcium absorption in a vitamin D receptor dependent but transcellular pathway independent manner. Kidney Int. 2020;97(6):1164–80.
Matsui I, Hamano T, Mikami S, Inoue K, Shimomura A, Nagasawa Y, et al. Retention of fetuin-A in renal tubular lumen protects the kidney from nephrocalcinosis in rats. Am J Physiol Renal Physiol. 2013;304(6):F751–60.
Matsui I, Hamano T, Mikami S, Fujii N, Takabatake Y, Nagasawa Y, et al. Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int. 2009;75(9):915–28.
Yamashiro T, Kuge H, Zhang J, Honke K. Calcineurin mediates the angiotensin II-induced aldosterone synthesis in the adrenal glands by up-regulation of transcription of the CYP11B2 gene. J Biochem. 2010;148(1):115–23.
Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25(22):5305–16.
Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, et al. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79(1):89–96.
Matsui I, Oka T, Kusunoki Y, Mori D, Hashimoto N, Matsumoto A, et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int. 2018;94(1):60–71.
Choi SR, Lim JH, Kim MY, Hong YA, Chung BH, Chung S, et al. Cinacalcet improves endothelial dysfunction and cardiac hypertrophy in patients on hemodialysis with secondary hyperparathyroidism. Nephron Clin Pract. 2012;122(1–2):1–8.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63.
Repo JM, Rantala IS, Honkanen TT, Mustonen JT, Koobi P, Tahvanainen AM, et al. Paricalcitol aggravates perivascular fibrosis in rats with renal insufficiency and low calcitriol. Kidney Int. 2007;72(8):977–84.
Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160(1):209–18.
Fuentes JJ, Pritchard MA, Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 1997;44(3):358–61.
Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114(11):1159–68.
Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, et al. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114(8):1058–71.
Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441(1):61–76.
Zhao XY, Eccleshall TR, Krishnan AV, Gross C, Feldman D. Analysis of vitamin D analog-induced heterodimerization of vitamin D receptor with retinoid X receptor using the yeast two-hybrid system. Mol Endocrinol. 1997;11(3):366–78.
Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–50.
Uchiyama Y, Higuchi Y, Takeda S, Masaki T, Shira-Ishi A, Sato K, et al. ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone. 2002;30(4):582–8.
Cannella G, Paoletti E, Delfino R, Peloso G, Molinari S, Traverso GB. Regression of left ventricular hypertrophy in hypertensive dialyzed uremic patients on long-term antihypertensive therapy. Kidney Int. 1993;44(4):881–6.
Pascual J, Berger SP, Chadban SJ, Citterio F, Kamar N, Hesselink DA, et al. Evidence-based practice: guidance for using everolimus in combination with low-exposure calcineurin inhibitors as initial immunosuppression in kidney transplant patients. Transplant Rev (Orlando). 2019;33(4):191–9.
Song YH, Cai GY, Xiao YF, Wang YP, Yuan BS, Xia YY, et al. Efficacy and safety of calcineurin inhibitor treatment for IgA nephropathy: a meta-analysis. BMC Nephrol. 2017;18(1):61.
Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37(6):602–12.
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38.
Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124(17):1838–47.
Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle. 2019;10:1228–40.
Lemmila S, Saha H, Virtanen V, Ala-Houhala I, Pasternack A. Effect of intravenous calcitriol on cardiac systolic and diastolic function in patients on hemodialysis. Am J Nephrol. 1998;18(5):404–10.
Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33(1):73–81.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412.
McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108(4):465–74.
Acknowledgments
The authors would like to thank Ms. Naoko Horimoto for her technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (no.25870408) to KI, a grant from The Kidney Foundation, Japan (JKFB 13-36) to KI, and maxacalcitol (22-oxacalcitriol: OCT) was kindly provided by Chugai Pharmaceutical Co., Ltd.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (no.25870408) to KI, a grant from The Kidney Foundation, Japan (JKFB 13–36) to KI and grants from Chugai Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kazunori Inoue, Isao Matsui, Keiji Okuda, Yasumasa Tsukamoto, Ayumi Matsumoto, Karin Shimada, Seiichi Yasuda, Yusuke Katsuma, Masaru Tanaka, Noriko Tanaka, and Takayuki Hamano. The first draft of the manuscript was written by Kazunori Inoue and Isao Matsui and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The Department of Inter-Organ Communication Research in Kidney Disease, Osaka University Graduate School of Medicine, received grants from Chugai Pharmaceutical Co., Ltd. The other authors have no conflicts of interest.
Ethics Approval
This retrospective cohort study was approved by the Ethics Committee (approval number: 2020-02) at Tanaka Kitanoda Hospital (Sakai-ku, Osaka, Japan).
Consent to Participate
Patients who did not declare refusal to participation according to opt-out policy were enrolled in this retrospective study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inoue, K., Matsui, I., Hamano, T. et al. Maxacalcitol (22-Oxacalcitriol (OCT)) Retards Progression of Left Ventricular Hypertrophy with Renal Dysfunction Through Inhibition of Calcineurin-NFAT Activity. Cardiovasc Drugs Ther 35, 381–397 (2021). https://doi.org/10.1007/s10557-020-07111-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07111-9