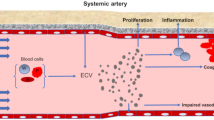
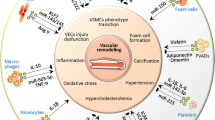
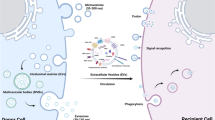
Abstract
Hypertension, a chronic and progressive disease, is an outstanding public health issue that affects nearly 40% of the adults worldwide. The increasing prevalence of hypertension is one of the leading causes of cardiovascular morbidity and mortality. Despite of the available treatment medications, an increasing number of hypertensive individuals continues to have uncontrolled blood pressure. In the vasculature, endothelial cells, vascular smooth muscle cells (VSMCs), and adventitial fibroblasts play a fundamental role in vascular homeostasis. The aberrant interactions between vascular cells might lead to hypertension and vascular remodeling. Identification of the precise mechanisms of vascular remodeling may be highly required to develop effective therapeutic approaches for hypertension. Recently, extracellular vesicle-mediated transfer of proteins or noncoding RNAs (ncRNAs) between vascular cells holds promise for the treatment of hypertension. Especially, extracellular vesicle-packaging ncRNAs have gained enormous attention of basic and clinical scientists because of their tremendous potential to act as novel clinical biomarkers and therapeutic targets of hypertension. Here we will discuss the current findings focusing on the emerging roles of extracellular vesicle-carrying ncRNAs in the pathologies of hypertension and its associated vascular remodeling. Furthermore, we will highlight the potential of extracellular vesicles and ncRNAs as biomarkers and therapeutic targets for hypertension. The future research directions on the challenges and perspectives of extracellular vesicles and ncRNAs in hypertensive vascular remodeling are also proposed.




Similar content being viewed by others
References
Jusic A, Devaux Y. Noncoding RNAs in hypertension. Hypertension (Dallas, Tex : 1979). 2019;74(3):477–92. https://doi.org/10.1161/hypertensionaha.119.13412.
Adler AJ, Prabhakaran D, Bovet P, Kazi DS, Mancia G, Mungal-Singh V, et al. Reducing cardiovascular mortality through prevention and management of raised blood pressure: a world heart federation roadmap. Glob Heart. 2015;10(2):111–22. https://doi.org/10.1016/j.gheart.2015.04.006.
Sekar D, Shilpa BR, Das AJ. Relevance of microRNA 21 in different types of hypertension. Curr Hypertens Rep. 2017;19(7):57. https://doi.org/10.1007/s11906-017-0752-z.
Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101(3):329–35. https://doi.org/10.1161/01.cir.101.3.329.
Leimena C, Qiu H. Non-coding RNA in the pathogenesis, progression and treatment of hypertension. International journal of molecular sciences. 2018;19(4). https://doi.org/10.3390/ijms19040927.
Marteau JB, Zaiou M, Siest G, Visvikis-Siest S. Genetic determinants of blood pressure regulation. J Hypertens. 2005;23(12):2127–43. https://doi.org/10.1097/01.hjh.0000186024.12364.2e.
Simon PH, Sylvestre MP, Tremblay J, Hamet P. Key considerations and methods in the study of gene-environment interactions. Am J Hypertens. 2016;29(8):891–9. https://doi.org/10.1093/ajh/hpw021.
Derhaschnig U, Testori C, Riedmueller E, Aschauer S, Wolzt M, Jilma B. Hypertensive emergencies are associated with elevated markers of inflammation, coagulation, platelet activation and fibrinolysis. J Hum Hypertens. 2013;27(6):368–73. https://doi.org/10.1038/jhh.2012.53.
Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension (Dallas, Tex : 1979). 2004;43(2):169–75. https://doi.org/10.1161/01.hyp.0000103160.35395.9e.
Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, et al. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46(3):518–23. https://doi.org/10.1016/j.jacc.2005.04.040.
Ebrahim S, Smith GD. Lowering blood pressure: a systematic review of sustained effects of non-pharmacological interventions. J Public Health Med. 1998;20(4):441–8. https://doi.org/10.1093/oxfordjournals.pubmed.a024800.
Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69(5):486–93. https://doi.org/10.1016/j.jacc.2016.10.077.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension (Dallas, Tex : 1979). 2003;42(6):1206–52. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
Turner JM, Kodali R. Should angiotensin-converting enzyme inhibitors ever be used for the management of hypertension? Curr Cardiol Rep. 2020;22(9):95. https://doi.org/10.1007/s11886-020-01352-8.
Ahluwalia M, Bangalore S. Management of hypertension in 2017: targets and therapies. Curr Opin Cardiol. 2017;32(4):413–21. https://doi.org/10.1097/hco.0000000000000408.
Mann SJ. Redefining beta-blocker use in hypertension: selecting the right beta-blocker and the right patient. Journal of the American Society of Hypertension : JASH. 2017;11(1):54–65. https://doi.org/10.1016/j.jash.2016.11.007.
Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension (Dallas, Tex : 1979). 2004;43(1):10–7. https://doi.org/10.1161/01.hyp.0000103630.72812.10.
Kim HW, Belin de Chantemele EJ, Weintraub NL. Perivascular adipocytes in vascular disease. Arterioscler Thromb Vasc Biol. 2019;39(11):2220–7. https://doi.org/10.1161/atvbaha.119.312304.
Brown IAM, Diederich L, Good ME, DeLalio LJ, Murphy SA, Cortese-Krott MM, et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler Thromb Vasc Biol. 2018;38(9):1969–85. https://doi.org/10.1161/atvbaha.118.311229.
Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116(6):1007–21. https://doi.org/10.1161/circresaha.116.303596.
Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–8. https://doi.org/10.1056/nejm199405193302008.
Sun HJ, Liu TY, Zhang F, Xiong XQ, Wang JJ, Chen Q, et al. Salusin-beta contributes to vascular remodeling associated with hypertension via promoting vascular smooth muscle cell proliferation and vascular fibrosis. Biochim Biophys Acta. 2015;1852(9):1709–18. https://doi.org/10.1016/j.bbadis.2015.05.008.
Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ. et al., NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. 2017;8(10):e3074. https://doi.org/10.1038/cddis.2017.470.
Lu QB, Wang HP, Tang ZH, Cheng H, Du Q, Wang YB, et al. Nesfatin-1 functions as a switch for phenotype transformation and proliferation of VSMCs in hypertensive vascular remodeling. Biochimica et biophysica acta Molecular basis of disease. 2018;1864(6 Pt A):2154–68. https://doi.org/10.1016/j.bbadis.2018.04.002.
Sun HJ, Wu ZY, Nie XW, Bian JS. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568. https://doi.org/10.3389/fphar.2019.01568.
Sun HJ, Hou B, Wang X, Zhu XX, Li KX, Qiu LY. Endothelial dysfunction and cardiometabolic diseases: role of long non-coding RNAs. Life Sci. 2016;167:6–11. https://doi.org/10.1016/j.lfs.2016.11.005.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40. https://doi.org/10.1007/5584_2016_90.
Sun HJ. Current opinion for hypertension in renal fibrosis. Adv Exp Med Biol. 2019;1165:37–47. https://doi.org/10.1007/978-981-13-8871-2_3.
Gumprecht J, Domek M, GYH L. Invited review: hypertension and atrial fibrillation: epidemiology, pathophysiology, and implications for management. 2019;33(12):824–36. https://doi.org/10.1038/s41371-019-0279-7.
Chang W, Wang J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells. 2019;8(8). https://doi.org/10.3390/cells8080853.
Erdbrugger U, Le TH. Extracellular vesicles as a novel diagnostic and research tool for patients with HTN and kidney disease. American journal of physiology Renal physiology. 2019;317(3):F641–f7. https://doi.org/10.1152/ajprenal.00071.2019.
Su SA, Xie Y, Fu Z, Wang Y, Wang JA, Xiang M. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget. 2017;8(15):25700–12. https://doi.org/10.18632/oncotarget.14878.
Heo J, Yang HC, Rhee WJ. Vascular smooth muscle cell-derived exosomal microRNAs regulate endothelial cell migration under PDGF stimulation. 2020;9(3). https://doi.org/10.3390/cells9030639.
Tong Y, Ye C, Ren XS, Qiu Y, Zang YH, Xiong XQ, et al. Exosome-mediated transfer of ACE (angiotensin-converting enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension (Dallas, Tex : 1979). 2018;72(4):881–8. https://doi.org/10.1161/hypertensionaha.118.11375.
Zhao L, Luo H, Li X, Li T, He J, Qi Q, et al. Exosomes derived from human pulmonary artery endothelial cells shift the balance between proliferation and apoptosis of smooth muscle cells. Cardiology. 2017;137(1):43–53. https://doi.org/10.1159/000453544.
Li S, Gao Y, Liu Y, Li J, Yang X, Hu R, et al. Myofibroblast-derived Exosomes contribute to development of a susceptible substrate for atrial fibrillation. Cardiology. 2020;145(5):324–32. https://doi.org/10.1159/000505641.
Dautova Y, Kapustin AN, Pappert K, Epple M, Okkenhaug H, Cook SJ, et al. Calcium phosphate particles stimulate interleukin-1β release from human vascular smooth muscle cells: a role for spleen tyrosine kinase and exosome release. J Mol Cell Cardiol. 2018;115:82–93. https://doi.org/10.1016/j.yjmcc.2017.12.007.
Wu Q, Yuan X. Differential miRNA expression analysis of extracellular vesicles from brain microvascular pericytes in spontaneous hypertensive rats. 2020;42(3):389–401. https://doi.org/10.1007/s10529-019-02788-x.
Liu X, Yuan W, Yang L, Li J, Cai J. miRNA profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res. 2019;12(1):75–83. https://doi.org/10.1007/s12265-017-9784-7.
Biró O, Alasztics B, Molvarec A, Joó J, Nagy B, Rigó J Jr. Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy hypertension. 2017;10:207–12. https://doi.org/10.1016/j.preghy.2017.09.002.
Omura J, Habbout K, Shimauchi T, Wu WH, Breuils-Bonnet S, Tremblay E, et al. Identification of the long non-coding RNA H19 as a new biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation. 2020. https://doi.org/10.1161/circulationaha.120.047626.
Li P, Yan X, Xu G, Pang Z, Weng J, Yin J, et al. A novel plasma lncRNA ENST00000416361 is upregulated in coronary artery disease and is related to inflammation and lipid metabolism. Mol Med Rep. 2020;21(6):2375–84. https://doi.org/10.3892/mmr.2020.11067.
Wu N, Jin L, Cai J. Profiling and bioinformatics analyses reveal differential circular RNA expression in hypertensive patients. Clinical and experimental hypertension (New York, NY : 1993). 2017;39(5):454–9. https://doi.org/10.1080/10641963.2016.1273944.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. https://doi.org/10.1038/nrm.2017.125.
Li M, Qian M, Kyler K, Xu J. Endothelial-vascular smooth muscle cells interactions in atherosclerosis. Frontiers in cardiovascular medicine. 2018;5:151. https://doi.org/10.3389/fcvm.2018.00151.
Ren XS, Tong Y, Qiu Y, Ye C, Wu N, Xiong XQ, et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. 2020;9(1):1698795. https://doi.org/10.1080/20013078.2019.1698795.
Karpman D, Stahl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13(9):545–62. https://doi.org/10.1038/nrneph.2017.98.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. https://doi.org/10.1146/annurev-cellbio-101512-122326.
ELA S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–57. https://doi.org/10.1038/nrd3978.
Dear JW, Street JM, Bailey MA. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics. 2013;13(10–11):1572–80. https://doi.org/10.1002/pmic.201200285.
Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–99. https://doi.org/10.1002/pmic.200800109.
Raimondo F, Morosi L, Chinello C, Magni F, Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011;11(4):709–20. https://doi.org/10.1002/pmic.201000422.
Sun HJ, Zhu XX, Cai WW, Qiu LY. Functional roles of exosomes in cardiovascular disorders: a systematic review. European review for medical and pharmacological sciences. 2017;21(22):5197–206. https://doi.org/10.26355/eurrev_201711_13840.
Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126(4):1152–62. https://doi.org/10.1172/jci81129.
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015;4:27066. https://doi.org/10.3402/jev.v4.27066.
Ghafarian F, Pashirzad M, Khazaei M. The clinical impact of exosomes in cardiovascular disorders: from basic science to clinical application. 2019;234(8):12226–36. https://doi.org/10.1002/jcp.27964.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. https://doi.org/10.1038/nri855.
Chen J, Hu C, Pan P. Extracellular vesicle microRNA transfer in lung diseases. Front Physiol. 2017;8:1028. https://doi.org/10.3389/fphys.2017.01028.
van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. https://doi.org/10.1124/pr.112.005983.
Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–64.
Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. The Keio journal of medicine. 2004;53(4):210–30. https://doi.org/10.2302/kjm.53.210.
Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009;102(4):711–8. https://doi.org/10.1160/th09-04-243.
Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694. https://doi.org/10.1371/journal.pone.0003694.
Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6(1):21–9. https://doi.org/10.1038/nrrheum.2009.229.
Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2015;24(4):199–206. https://doi.org/10.1016/j.carpath.2015.04.007.
Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017;13(12):731–49. https://doi.org/10.1038/nrneph.2017.148.
Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 2015;34(4):474–90. https://doi.org/10.1002/mas.21420.
Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–81. https://doi.org/10.1016/j.ceb.2009.03.007.
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Journal of extracellular vesicles. 2014;3:26913. https://doi.org/10.3402/jev.v3.26913.
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of extracellular vesicles. 2013;2. https://doi.org/10.3402/jev.v2i0.20360.
Bei Y, Das S, Rodosthenous RS, Holvoet P, Vanhaverbeke M, Monteiro MC, et al. Extracellular vesicles in cardiovascular theranostics. Theranostics. 2017;7(17):4168–82. https://doi.org/10.7150/thno.21274.
Krohn JB, Hutcheson JD, Martinez-Martinez E, Aikawa E. Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J Physiol. 2016;594(11):2895–903. https://doi.org/10.1113/jp271338.
Jansen F, Nickenig G, Werner N. Extracellular vesicles in cardiovascular disease: potential applications in diagnosis, prognosis, and epidemiology. Circ Res. 2017;120(10):1649–57. https://doi.org/10.1161/circresaha.117.310752.
Blaser MC, Aikawa E. Roles and regulation of extracellular vesicles in cardiovascular mineral metabolism. Frontiers in cardiovascular medicine. 2018;5:187. https://doi.org/10.3389/fcvm.2018.00187.
Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–8. https://doi.org/10.1038/nature11233.
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89. https://doi.org/10.1101/gr.132159.111.
Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2(12):919–29. https://doi.org/10.1038/35103511.
Siebert V, Allencherril J, Ye Y, Wehrens XHT, Birnbaum Y. The role of non-coding RNAs in ischemic myocardial reperfusion injury. Cardiovasc Drugs Ther. 2019;33(4):489–98. https://doi.org/10.1007/s10557-019-06893-x.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74. https://doi.org/10.1038/nrg3074.
Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011;109(3):334–47. https://doi.org/10.1161/circresaha.110.228676.
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46. https://doi.org/10.1172/jci70577.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. https://doi.org/10.1016/s0092-8674(04)00045-5.
Fukaya T, Tomari Y. MicroRNAs mediate gene silencing via multiple different pathways in drosophila. Mol Cell. 2012;48(6):825–36. https://doi.org/10.1016/j.molcel.2012.09.024.
Gu S, Kay MA. How do miRNAs mediate translational repression? Silence. 2010;1(1):11. https://doi.org/10.1186/1758-907x-1-11.
Boateng E, Krauss-Etschmann S. miRNAs in lung development and diseases. International journal of molecular sciences. 2020;21(8). https://doi.org/10.3390/ijms21082765.
Marques FZ, Booth SA, Charchar FJ. The emerging role of non-coding RNA in essential hypertension and blood pressure regulation. J Hum Hypertens. 2015;29(8):459–67. https://doi.org/10.1038/jhh.2014.99.
Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–61. https://doi.org/10.1016/j.tcb.2011.04.001.
Lorenzen JM, Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12(6):360–73. https://doi.org/10.1038/nrneph.2016.51.
Gomes CPC, Salgado-Somoza A, Creemers EE, Dieterich C, Lustrek M, Devaux Y. Circular RNAs in the cardiovascular system. Non-coding RNA research. 2018;3(1):1–11. https://doi.org/10.1016/j.ncrna.2018.02.002.
Bayoumi AS, Aonuma T, Teoh JP, Tang YL, Kim IM. Circular noncoding RNAs as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol Sin. 2018;39(7):1100–9. https://doi.org/10.1038/aps.2017.196.
Devaux Y. Transcriptome of blood cells as a reservoir of cardiovascular biomarkers. Biochimica et biophysica acta Molecular cell research. 2017;1864(1):209–16. https://doi.org/10.1016/j.bbamcr.2016.11.005.
Ding S, Zhu Y, Liang Y, Huang H, Xu Y, Zhong C. Circular RNAs in vascular functions and diseases. Adv Exp Med Biol. 2018;1087:287–97. https://doi.org/10.1007/978-981-13-1426-1_23.
Wang YF, Lian XL, Zhong JY, Su SX, Xu YF, Xie XF, et al. Serum exosomal microRNA let-7i-3p as candidate diagnostic biomarker for Kawasaki disease patients with coronary artery aneurysm. 2019;71(7):891–900. https://doi.org/10.1002/iub.2015.
Hough KP, Trevor JL, Strenkowski JG, Wang Y, Chacko BK, Tousif S, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64. https://doi.org/10.1016/j.redox.2018.06.009.
Ghoshal P, Singla B, Lin H, Cherian-Shaw M, Tritz R, Padgett CA, et al. Loss of GTPase activating protein neurofibromin stimulates paracrine cell communication via macropinocytosis. Redox Biol. 2019;27:101224. https://doi.org/10.1016/j.redox.2019.101224.
Xu MY, Ye ZS, Song XT, Huang RC. Differences in the cargos and functions of exosomes derived from six cardiac cell types: a systematic review. 2019;10(1):194. https://doi.org/10.1186/s13287-019-1297-7.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. Journal of extracellular vesicles. 2014;3. https://doi.org/10.3402/jev.v3.24641.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88. https://doi.org/10.1016/j.tcb.2016.11.003.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12. https://doi.org/10.1007/s10571-016-0366-z.
Li Z, Zhu X, Huang S. Extracellular vesicle long non-coding RNAs and circular RNAs: biology, functions and applications in cancer. Cancer Lett. 2020;489:111–20. https://doi.org/10.1016/j.canlet.2020.06.006.
Abramowicz A, Story MD. The long and short of it: the emerging roles of non-coding RNA in small extracellular vesicles. 2020;12(6). https://doi.org/10.3390/cancers12061445.
Wang M, Zhou L, Yu F, Zhang Y, Li P, Wang K. The functional roles of exosomal long non-coding RNAs in cancer. Cellular and molecular life sciences : CMLS. 2019;76(11):2059–76. https://doi.org/10.1007/s00018-019-03018-3.
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. https://doi.org/10.1038/ncomms3980.
Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. https://doi.org/10.1186/1471-2164-13-357.
Rotllan N, Price N, Pati P, Goedeke L, Fernández-Hernando C. microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis. 2016;246:352–60. https://doi.org/10.1016/j.atherosclerosis.2016.01.025.
Barros ER, Carvajal CA. Urinary exosomes and their cargo: potential biomarkers for mineralocorticoid arterial hypertension? Front Endocrinol. 2017;8:230. https://doi.org/10.3389/fendo.2017.00230.
Esteva-Font C, Wang X, Ars E, Guillen-Gomez E, Sans L, Gonzalez Saavedra I, et al. Are sodium transporters in urinary exosomes reliable markers of tubular sodium reabsorption in hypertensive patients? Nephron Physiology. 2010;114(3):p25–34. https://doi.org/10.1159/000274468.
Qi Y, Wang X, Rose KL, MacDonald WH, Zhang B, Schey KL, et al. Activation of the endogenous renin-angiotensin-aldosterone system or aldosterone administration increases urinary exosomal sodium channel excretion. Journal of the American Society of Nephrology : JASN. 2016;27(2):646–56. https://doi.org/10.1681/asn.2014111137.
Gonzalez-Calero L, Martinez PJ, Martin-Lorenzo M, Baldan-Martin M, Ruiz-Hurtado G, de la Cuesta F, et al. Urinary exosomes reveal protein signatures in hypertensive patients with albuminuria. Oncotarget. 2017;8(27):44217–31. https://doi.org/10.18632/oncotarget.17787.
Jayaseelan VP, Arumugam P. Exosomal microRNAs as a promising theragnostic tool for essential hypertension. Hypertension research : official journal of the Japanese Society of Hypertension. 2020;43(1):74–5. https://doi.org/10.1038/s41440-019-0343-2.
Perez-Hernandez J, Olivares D, Forner MJ, Ortega A, Solaz E, Martinez F, et al. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. 2018;16(1):228. https://doi.org/10.1186/s12967-018-1604-6.
Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6(6):499–506. https://doi.org/10.1038/ncb1137.
Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation. 2015;131(24):2120–30. https://doi.org/10.1161/circulationaha.115.015687.
Guescini M, Leo G, Genedani S, Carone C, Pederzoli F, Ciruela F, et al. Microvesicle and tunneling nanotube mediated intercellular transfer of g-protein coupled receptors in cell cultures. Exp Cell Res. 2012;318(5):603–13. https://doi.org/10.1016/j.yexcr.2012.01.005.
Cambier L, Giani JF, Liu W, Ijichi T, Echavez AK, Valle J, et al. Angiotensin II-induced end-organ damage in mice is attenuated by human exosomes and by an exosomal Y RNA fragment. Hypertension (Dallas, Tex : 1979). 2018;72(2):370–80. https://doi.org/10.1161/hypertensionaha.118.11239.
Gao Y, Chen T, Raj JU. Endothelial and smooth muscle cell interactions in the pathobiology of pulmonary hypertension. Am J Respir Cell Mol Biol. 2016;54(4):451–60. https://doi.org/10.1165/rcmb.2015-0323TR.
Shin WS, Hemmi C, Toyo-oka T. Co-culture and crosstalk between endothelial cells and vascular smooth muscle cells mediated by intracellular calcium. Methods in molecular biology (Clifton, NJ). 2002:188, 347–57. https://doi.org/10.1385/1-59259-185-x:347.
Humbert M, Montani D, Perros F, Dorfmuller P, Adnot S, Eddahibi S. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vasc Pharmacol. 2008;49(4–6):113–8. https://doi.org/10.1016/j.vph.2008.06.003.
de la Cuesta F, Passalacqua I, Rodor J, Bhushan R, Denby L, Baker AH. Extracellular vesicle cross-talk between pulmonary artery smooth muscle cells and endothelium during excessive TGF-β signalling: implications for PAH vascular remodelling. Cell communication and signaling : CCS. 2019;17(1):143. https://doi.org/10.1186/s12964-019-0449-9.
Wang YN, Shan K, Yao MD, Yao J, Wang JJ, Li X, et al. Long noncoding RNA-GAS5: a novel regulator of hypertension-induced vascular remodeling. Hypertension (Dallas, Tex : 1979). 2016;68(3):736–48. https://doi.org/10.1161/hypertensionaha.116.07259.
Zhou J, Li YS, Nguyen P, Wang KC, Weiss A, Kuo YC, et al. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113(1):40–51. https://doi.org/10.1161/circresaha.113.280883.
Jansen F, Stumpf T, Proebsting S, Franklin BS, Wenzel D, Pfeifer P, et al. Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. J Mol Cell Cardiol. 2017;104:43–52. https://doi.org/10.1016/j.yjmcc.2016.12.005.
Gu J, Zhang H, Ji B, Jiang H, Zhao T, Jiang R, et al. Vesicle miR-195 derived from endothelial cells inhibits expression of serotonin transporter in vessel smooth muscle cells. Sci Rep. 2017;7:43546. https://doi.org/10.1038/srep43546.
Lin X, He Y, Hou X, Zhang Z, Wang R, Wu Q. Endothelial cells can regulate smooth muscle cells in contractile phenotype through the miR-206/ARF6&NCX1/exosome axis. PLoS One. 2016;11(3):e0152959. https://doi.org/10.1371/journal.pone.0152959.
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–56. https://doi.org/10.1038/ncb2441.
Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, et al. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Molecular therapy : the journal of the American Society of Gene Therapy. 2017;25(6):1279–94. https://doi.org/10.1016/j.ymthe.2017.03.031.
Dou YQ, Kong P, Li CL, Sun HX, Li WW, Yu Y, et al. Smooth muscle SIRT1 reprograms endothelial cells to suppress angiogenesis after ischemia. Theranostics. 2020;10(3):1197–212. https://doi.org/10.7150/thno.39320.
Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114(1):67–78. https://doi.org/10.1161/circresaha.114.301633.
Bienertova-Vasku J, Novak J, Vasku A. MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. Journal of the American Society of Hypertension : JASH. 2015;9(3):221–34. https://doi.org/10.1016/j.jash.2014.12.011.
Ling L, Chen D, Tong Y, Zang YH, Ren XS, Zhou H, et al. Fibronectin type III domain containing 5 attenuates NLRP3 inflammasome activation and phenotypic transformation of adventitial fibroblasts in spontaneously hypertensive rats. J Hypertens. 2018;36(5):1104–14. https://doi.org/10.1097/hjh.0000000000001654.
Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Advances in pharmacology (San Diego, Calif). 2018;81:241–330. https://doi.org/10.1016/bs.apha.2017.08.002.
Wu N, Ye C, Zheng F, Wan GW, Wu LL, Chen Q, et al. MiR155-5p inhibits cell migration and oxidative stress in vascular smooth muscle cells of spontaneously hypertensive rats. 2020;9(3). https://doi.org/10.3390/antiox9030204.
Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31(12):1247–54. https://doi.org/10.1093/ajh/hpy148.
Tipton AJ, Sullivan JC. Sex differences in T cells in hypertension. Clin Ther. 2014;36(12):1882–900. https://doi.org/10.1016/j.clinthera.2014.07.011.
Muiesan ML, Salvetti M, Rosei CA, Paini A. Gender differences in antihypertensive treatment: myths or legends? High blood pressure & cardiovascular prevention: the official journal of the Italian Society of Hypertension. 2016;23(2):105–13. https://doi.org/10.1007/s40292-016-0148-1.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama. 2014;311(5):507–20. https://doi.org/10.1001/jama.2013.284427.
Perumareddi P. Prevention of hypertension related to cardiovascular disease. Primary care. 2019;46(1):27–39. https://doi.org/10.1016/j.pop.2018.10.005.
Harischandra H, Yuan W, Loghry HJ. Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex-specific differences in cargo and a sensitivity to ivermectin. 2018;12(4):e0006438. https://doi.org/10.1371/journal.pntd.0006438.
Bilal U, Diez-Roux AV. Troubling trends in health disparities. N Engl J Med. 2018;378(16):1557–8. https://doi.org/10.1056/NEJMc1800328.
Noren Hooten N, McFarland MH, Freeman DW, Mode NA, Ezike N, Zonderman AB, et al. Association of extracellular vesicle protein cargo with race and clinical markers of mortality. 2019;9(1):17582. https://doi.org/10.1038/s41598-019-53640-1.
Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020;11(1):3. https://doi.org/10.1186/s13287-019-1508-2.
Dekker M, Waissi F, van Bennekom J, Silvis MJM, Timmerman N, Bank IEM, et al. Plasma extracellular vesicle proteins are associated with stress-induced myocardial ischemia in women presenting with chest pain. Sci Rep. 2020;10(1):12257. https://doi.org/10.1038/s41598-020-69297-0.
Silver JL, Alexander SE. Extracellular vesicular miRNA expression is not a proxy for skeletal muscle miRNA expression in males and females following acute, moderate intensity exercise. 2020;8(16):e14520. https://doi.org/10.14814/phy2.14520.
Wallace E, Morrell NW, Yang XD, Long L, Stevens H, Nilsen M, et al. A sex-specific MicroRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med. 2015;191(12):1432–42. https://doi.org/10.1164/rccm.201412-2148OC.
Florijn BW, Bijkerk R, van der Veer EP, van Zonneveld AJ. Gender and cardiovascular disease: are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc Res. 2018;114(2):210–25. https://doi.org/10.1093/cvr/cvx223.
Li H, Ouyang Y, Sadovsky E, Parks WT, Chu T, Sadovsky Y. Unique microRNA signals in plasma exosomes from pregnancies complicated by preeclampsia. Hypertension (Dallas, Tex : 1979). 2020;(3):75, 762–771. https://doi.org/10.1161/hypertensionaha.119.14081.
Aryan L, Medzikovic L, Umar S, Eghbali M. Pregnancy-associated cardiac dysfunction and the regulatory role of microRNAs. Biol Sex Differ. 2020;11(1):14. https://doi.org/10.1186/s13293-020-00292-w.
Qin S, Predescu DN, Patel M, Drazkowski P, Ganesh B, Predescu SA. Sex differences in the proliferation of pulmonary artery endothelial cells: implications for plexiform arteriopathy. 2020;(9):133. https://doi.org/10.1242/jcs.237776.
Ahmad T, Miller PE, McCullough M, Desai NR, Riello R, Psotka M, et al. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur J Heart Fail. 2019;21(9):1064–78. https://doi.org/10.1002/ejhf.1557.
Douguet D, Patel A, Xu A, Vanhoutte PM, Honoré E. Piezo ion channels in cardiovascular mechanobiology. Trends Pharmacol Sci. 2019;40(12):956–70. https://doi.org/10.1016/j.tips.2019.10.002.
Qi YX, Han Y, Jiang ZL. Mechanobiology and vascular remodeling: from membrane to nucleus. Adv Exp Med Biol. 2018;1097:69–82. https://doi.org/10.1007/978-3-319-96445-4_4.
Randhawa PK, Jaggi AS. TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res Cardiol. 2015;110(6):54. https://doi.org/10.1007/s00395-015-0512-7.
McSweeney SR, Warabi E, Siow RC. Nrf2 as an endothelial mechanosensitive transcription factor: going with the flow. Hypertension (Dallas, Tex : 1979). 2016;67(1):20–9. https://doi.org/10.1161/hypertensionaha.115.06146.
Good ME, Musante L, La Salvia S, Howell NL, Carey RM, Le TH, et al. Circulating extracellular vesicles in normotension restrain vasodilation in resistance arteries. Hypertension (Dallas, Tex : 1979). 2020;75(1):218–28. https://doi.org/10.1161/hypertensionaha.119.13363.
Akerman AW, Blanding WM, Stroud RE, Nadeau EK, Mukherjee R, Ruddy JM, et al. Elevated wall tension leads to reduced miR-133a in the thoracic aorta by exosome release. J Am Heart Assoc. 2019;8(1):e010332. https://doi.org/10.1161/jaha.118.010332.
Funding
This work was supported by grants from the Fund of the National Natural Science Foundation of China (81700364), Jiangsu Natural Science Foundation (BK20170179, BK20191138), Jiangsu Province Department of Science and Technology (BE2020634), Key Young Medical Talent Project of Jiangsu Health Commission (QNRC2016158), Project funded by China Postdoctoral Science Foundation (2017M611688), and Project funded by Jiangsu Postdoctoral Science Foundation (1701062C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, JR., Sun, HJ. Extracellular Vesicle-Mediated Vascular Cell Communications in Hypertension: Mechanism Insights and Therapeutic Potential of ncRNAs. Cardiovasc Drugs Ther 36, 157–172 (2022). https://doi.org/10.1007/s10557-020-07080-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07080-z