Abstract
Purpose
Atherosclerosis, a chronic disease of the arteries, results from pathological processes including the accumulation and aggregation of oxidized low-density lipoprotein (oxLDL) in the vessel walls, development of neointima, formation of a fibrous cap, and migration of immune cells to damaged vascular endothelium. Recent studies have shown that mitochondrial dysfunction is closely associated with the development and progression of atherosclerosis. Idebenone, a short-chain benzoquinone similar in structure to coenzyme Q10, can effectively clear oxygen free radicals as an electron carrier and antioxidant. In the present study, we aim to investigate weather idebenone protects against atherosclerosis in apolipoprotein E-deficient (apoE−/−) mice.
Methods
apoE−/− mice receiving a high-fat diet (HFD) were treated with idebenone for 16 weeks. A total of 60 mice were randomized into the following four groups: (1) HFD, (2) HFD and low-dose idebenone (100 mg/kg/d), (3) HFD and medium-dose idebenone (200 mg/kg/d), and (4) HFD and high-dose (400 mg/kg/d). Proteomic analysis was performed between the HFD and idebenone-high-dose group. Plaque analysis was carried out by histological and immunohistochemical staining. Western blot, TUNEL staining, and MitoSOX assays were performed in human umbilical vein endothelial cells (HUVECs) to investigate the SIRT3-SOD2-mtROS pathway.
Results
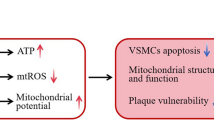
Histological and morphological analysis demonstrated that idebenone significantly reduced plaque burden and plaque size. Idebenone treatment effectively stabilized the atherosclerotic plaques. In mice treated with idebenone, 351 up-regulated and 379 down-regulated proteins were found to be significantly altered in proteomic analysis. In particular, the expression of SIRT3, SOD2, and NLRP3 was significantly regulated in the idebenone treatment groups compared with the HFD group both in vivo and in vitro. We further confirmed that idebenone protected against endothelial cell damage and inhibited the production of mitochondrial reactive oxygen species (mtROS) in cholesterol-treated HUVECs.
Conclusions
We demonstrated that idebenone acted as a mitochondrial protective agent by inhibiting the activation of NLPR3 via the SIRT3-SOD2-mtROS pathway. Idebenone may be a promising therapy for patients with atherosclerosis by improving mitochondrial dysfunction and inhibiting oxidative stress.







Similar content being viewed by others
Abbreviations
- LDL:
-
Low-density lipoprotein
- OxLDL:
-
Oxidized low-density lipoprotein
- HFD:
-
High-fat diet
- HUVECs:
-
Human umbilical vein endothelial cells
- ROS:
-
Reactive oxygen species
- mtROS:
-
Mitochondrial reactive oxygen species
- SIRT3:
-
Sirtuin3
- SOD2:
-
Superoxide dismutase 2
- CoQ10:
-
Coenzyme Q10
- ECM:
-
Endothelial cell medium
- ECGS:
-
Endothelial cell growth supplement
- CCK-8:
-
Cell counting kit-8
- IDE-L:
-
Low-dose idebenone group
- IDE-M:
-
Medium-dose idebenone group
- IDE-H:
-
High-dose idebenone group
- OCT:
-
Optimal cutting temperature
- RIPA:
-
Radioimmunoprecipitation assay
- H&E:
-
Hematoxylin and eosin
- SMCs:
-
Smooth muscle cells
- TNF-α:
-
Tumor necrosis factor-α
- PBS:
-
Phosphate-buffered saline
- DAB:
-
Diaminobenzidine
- TEAB:
-
Triethylamine-carbonic acid buffer
- MMP:
-
Mitochondrial membrane potential
- MDA:
-
Malondialdehyde
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- MRC:
-
Mitochondrial respiratory chain
References
Pant S, Deshmukh A, Gurumurthy GS, Pothineni NV, Watts TE, Romeo F, et al. Inflammation and atherosclerosis--revisited. J Cardiovasc Pharmacol Ther. 2014;19(2):170–8.
Schaftenaar F, Frodermann V, Kuiper J, Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 2016;27(3):209–15.
Forstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017 Feb 17;120(4):713–35.
Sohrabi Y, Lagache SMM, Schnack L, Godfrey R, Kahles F, Bruemmer D, et al. mTOR-dependent oxidative stress regulates oxLDL-induced trained innate immunity in human monocytes. Front Immunol. 2019;9:3155.
Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594(3):509–25.
Yu EP, Bennett MR. Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol Metab. 2014;25(9):481–7.
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42.
Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31(2):113–26.
Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–90.
Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592(5):743–58.
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684–96.
Xiao M, Zhong H, Xia L, Tao Y, Yin H. Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic Biol Med. 2017;111:316–27.
Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, Orekhov AN. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med. 2018;50(2):121–7.
Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–60.
Tabares-Guevara JH, Lara-Guzmán OJ, Londoño-Londoño JA, Sierra JA, León-Varela YM, Álvarez-Quintero RM. Natural biflavonoids modulate macrophage-oxidized LDL interaction in vitro and promote atheroprotection in vivo. Front Immunol. 2017;8:923.
Montenegro L, Modica MN, Salerno L, Panico AM, Crasci L, Puglisi G, et al. In vitro antioxidant activity of Idebenone derivative-loaded solid lipid nanoparticles. Molecules. 2017;22(6).
Jaber S, Polster BM. Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier? J Bioenerg Biomembr. 2015;47(1–2):111–8.
Lin P, Liu J, Ren M, Ji K, Li L, Zhang B, et al. Idebenone protects against oxidized low density lipoprotein induced mitochondrial dysfunction in vascular endothelial cells via GSK3beta/beta-catenin signalling pathways. Biochem Biophys Res Commun. 2015;465(3):548–55.
Ma J, Qiao L, Meng L, Ma L, Zhao Y, Liu X, et al. Tongxinluo may stabilize atherosclerotic plaque via multiple mechanisms scanning by genechip. Biomed Pharmacother. 2019;113:108767.
Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 Inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6(9).
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–50.
Angajala A, Lim S, Phillips JB, Kim JH, Yates C, You Z, et al. Diverse roles of mitochondria in immune responses: novel insights into Immuno-metabolism. Front Immunol. 2018;9:1605.
Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106(5):544–9.
Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128(7):702–12.
Subramanian S, Kalyanaraman B, Migrino RQ. Mitochondrially targeted antioxidants for the treatment of cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2010;5(1):54–65.
Tiefenbach J, Magomedova L, Liu J, Reunov AA, Tsai R, Eappen NS, Jockusch RA, Nislow C, Cummins CL, Krause HM. Idebenone and coenzyme Q10 are novel PPARalpha/gamma ligands, with potential for treatment of fatty liver diseases. Dis Model Mech 2018;11(9).
Yan A, Liu Z, Song L, Wang X, Zhang Y, Wu N, et al. Idebenone alleviates Neuroinflammation and modulates microglial polarization in LPS-stimulated BV2 cells and MPTP-induced Parkinson's disease mice. Front Cell Neurosci. 2018;12:529.
Becker C, Bray-French K, Drewe J. Pharmacokinetic evaluation of idebenone. Expert Opin Drug Metab Toxicol. 2010;6(11):1437–44.
Cardoso SM, Pereira C, Oliveira R. Mitochondrial function is differentially affected upon oxidative stress. Free Radic Biol Med. 1999;26(1–2):3–13.
Giorgio V, Schiavone M, Galber C, Carini M, Da Ros T, Petronilli V, et al. The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects. Biochim Biophys Acta Bioenerg. 2018;1859(9):901–8.
Yu-Wai-Man P, Soiferman D, Moore DG, Burte F, Saada A. Evaluating the therapeutic potential of idebenone and related quinone analogues in Leber hereditary optic neuropathy. Mitochondrion. 2017;36:36–42.
Kobayashi T, Yoshida K, Mitani M, Torii H, Tanayama S. Metabolism of idebenone (CV-2619), a new cerebral metabolism improving agent: isolation and identification of metabolites in the rat and dog. Aust J Pharm. 1985;8(6):448–56.
Xu ZR, Li JY, Dong XW, Tan ZJ, Wu WZ, Xie QM, et al. Apple polyphenols decrease atherosclerosis and hepatic steatosis in ApoE−/− mice through the ROS/MAPK/NF-κB pathway. Nutrients. 2015;7(8):7085–105.
Gueven N. Idebenone for Leber's hereditary optic neuropathy. Drugs Today (Barc). 2016;52(3):173–81.
Fadda LM, Hagar H, Mohamed AM, Ali HM. Quercetin and Idebenone ameliorate oxidative stress, inflammation, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in rat liver. Dose Response. 2018;16(4):1559325818812188.
Traba J, Geiger SS, Kwarteng-Siaw M, Han K, Ra OH, Siegel RM, et al. Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3-mediated activation of superoxide dismutase 2. J Biol Chem. 2017;292(29):12153–64.
Kane AE, Sinclair DA. Sirtuins and NAD(+) in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123(7):868–85.
Sosnowska B, Mazidi M, Penson P, Gluba-Brzozka A, Rysz J, Banach M. The sirtuin family members SIRT1, SIRT3 and SIRT6: their role in vascular biology and atherogenesis. Atherosclerosis. 2017;265:275–82.
Wu J, Zeng Z, Zhang W, Deng Z, Wan Y, Zhang Y, et al. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free Radic Res. 2019;53(2):139–49.
Salvatori I, Valle C, Ferri A, Carri MT. SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem Int. 2017;109:184–92.
Karnewar S, Vasamsetti SB, Gopoju R, Kanugula AK, Ganji SK, Prabhakar S, et al. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci Rep. 2016;6:24108.
Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, et al. Sirt3 impairment and SOD2 Hyperacetylation in vascular oxidative stress and hypertension. Circ Res. 2017;121(5):564–74.
Haneklaus M, O'Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62.
Song N, Li T. Regulation of NLRP3 Inflammasome by phosphorylation. Front Immunol. 2018;9:2305.
Minutoli L, Puzzolo D, Rinaldi M, Irrera N, Marini H, Arcoraci V, et al. ROS-mediated NLRP3 Inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxidative Med Cell Longev. 2016;2016:2183026.
Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–59.
Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122(12):1722–40.
Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233(3):2116–32.
Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y, et al. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Investig. 2017;97(8):922–34.
Corrêa R, Silva LFF, Ribeiro DJS, Almeida RDN, Santos IO, Corrêa LH, et al. Lysophosphatidylcholine Induces NLRP3 Inflammasome-Mediated Foam Cell Formation and Pyroptosis inHuman Monocytes and Endothelial Cells. Front Immunol. 2020;10:2927.
Perry JB, Davis GN, Allen ME, Makrecka-Kuka M, Dambrova M, Grange RW, et al. Cardioprotective effects of idebenone do not involve ROS scavenging: evidence for mitochondrial complex I bypass in ischemia/reperfusion injury. J Mol Cell Cardiol. 2019;135:160–71.
Acknowledgments
We thank the State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine for appropriate advice about the animal experimental protocol. This manuscript was edited for English language by Medjaden Bioscience Limited. We thank Medjaden Bioscience Limited for English language editing.
Funding
This work was supported by the Taishan Scholars Program of Shandong Province and Policy supported projects of collaborative innovation and achievement transformation in universities and research institutes of Jinan (2019GXRC050).
Author information
Authors and Affiliations
Contributions
Wei Jiang, Fuchen Liu, Pengfei Lin, and Chuanzhu Yan were involved in the conceptualization and design of the study, acquisition and interpretation of data, drafting of the manuscript, and final approval of the version to be published.
Corresponding authors
Ethics declarations
Ethics Statement
This study was approved by the Brain Science Research Institute and the Ethics Committee from Qilu Hospital of Shandong University (Jinan, China).
Competing Interests
The authors declare that there are no known conflicts of interest associated with this publication, and no significant financial support was received for this work that could have influenced its outcome.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Fig. S1. Tissues between the aortic root and the abdominal aorta were selected for the proteomics. Fig. S2 - Fig. S4. GO enrichment bubble plot of differentially expressed proteins in three categories. Fig. S5 - Fig. S7. Comprehensive heat map for cluster analysis of the enrichment patterns of GO functional categories. Fig. S8. COG/KOG functional classification chart of differentially expressed proteins mainly involved in (C) energy production and conversion, (E) amino acid transport and metabolism, (I) lipid transport and metabolism, (R) general function prediction, and (T) signal transduction mechanisms. Fig. S9. Idebenone can suppress the expression of IL-1β tested by ELISA in both in vitro and in vivo experiments. (A) Quantification of the expression of IL-1β by ELISA in the four different treatment groups with apoE−/− mice (each group n = 7). (B) Quantification of the expression of IL-1β by ELISA in the four different groups (Si-control, Si-control + 10 μM CHOL, Si-control + 10 μM CHOL + 0.2 μM IDE, and Si-SIRT3 + 10 μM CHOL + 0.2 μM IDE) with HUVECs (each group n = 3). Representative data were from three independent experiments. Data are expressed as the mean ± SD. (A)*P < 0.05 vs. control; (B)*P < 0.05 vs.10 μM CHOL, #P < 0.05 vs. Si-control + 10 μM CHOL + 0.2 μM IDE. CHOL: cholesterol, IDE: idebenone. HFD: high-fat diet, L: low-dose, M: medium-dose, H: high-dose. Fig. S10. (a) Representative fluorescence image by MitoSOX and MitoTracker staining between control and 0.2 μM idebenone group (each group n = 5). (b) Quantitative analysis of intensity of red fluorescence by MitoSOX staining. (c) CCK8 detects the cell viability of HUVECs between control and 0.2 μM idebenone group (each group n = 5). Representative data were from three independent experiments. Data are expressed as the mean ± SD. IDE: idebenone. (DOCX 14586 kb)
Rights and permissions
About this article
Cite this article
Jiang, W., Geng, H., Lv, X. et al. Idebenone Protects against Atherosclerosis in Apolipoprotein E-Deficient Mice Via Activation of the SIRT3-SOD2-mtROS Pathway. Cardiovasc Drugs Ther 35, 1129–1145 (2021). https://doi.org/10.1007/s10557-020-07018-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07018-5