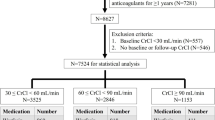
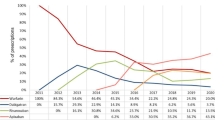
Abstract
The need for anticoagulation in patients with atrial fibrillation (AF) is fundamental to prevent thromboembolic events. Direct oral anticoagulants (DOACs) recently demonstrated to be superior, or at least equal, to Warfarin in reducing the risk for stroke/systemic embolism and preventing major bleeding and intracranial hemorrhages. The AF population often suffers from chronic kidney disease (CKD). Indeed, the relationship between AF and renal function is bidirectional: AF can trigger kidney failure, while kidney impairment can promote alterations able to enhance AF. Therefore, there are concerns regarding prescriptions of anticoagulants to patients with AF and CKD. The worsening in kidney function can be effectively due to anticoagulants administration. Warfarin has been recognized to promote acute kidney injury in case of excessive anticoagulation levels. Nevertheless, further mechanisms can induce the chronic worsening of renal function, thus leading to terminal kidney failure as observed in post-hoc analysis from registration trials and dedicated observational studies. By contrast, DOACs seem to protect kidneys from injuries more efficiently than Warfarin, although they still continue to play a role in promoting some kidney lesions. However, the exact mechanisms remain unknown. This narrative review aimed to discuss the influence of oral anticoagulants on renal impairment as well as to overview potential pathophysiological mechanisms related to this clinical complication.




Similar content being viewed by others
References
Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007;298:2038–47.
Huang SY, Chen YC, Kao YH, et al. Renal failure induces atrial arrhythmogenesis from discrepant electrophysiological remodeling and calcium regulation in pulmonary veins, sinoatrial node, and atria. Int J Cardiol. 2016;202:846–57.
Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482.
Cho SW, Hwang JK, Chun KJ, et al. Impact of moderate to severe renal impairment on long-term clinical outcomes in patients with atrial fibrillation. J Cardiol. 2017;69:577–83.
Genovesi S, Santoro A. Anticoagulants, renal failure and atrial fibrillation. Expert Opin Drug Saf. 2013;12:1–3.
Raccah BH, Perlman A, Danenberg HD, Pollak A, Muszkat M, Matok I. Major bleeding and Hemorrhagic stroke with direct Oral anticoagulants in patients with renal failure: systematic review and meta-analysis of randomized trials. Chest. 2016;149:1516–24.
Wong CX, Odutayo A, Emdin CA, Kinnear NJ, Sun MT. Meta-analysis of anticoagulation use, stroke, thromboembolism, bleeding, and mortality in patients with atrial fibrillation on Dialysis. Am J Cardiol. 2016;117:1934–41.
Harel Z, Mamdani M, Juurlink DN, et al. Novel oral anticoagulants and the risk of major hemorrhage in elderly patients with chronic kidney disease: a nested case-control study. Can J Cardiol. 2016;32:986.e17–22.
Fanola CL, Mooney D, Cowan AJ, et al. Incidence of severe renal dysfunction among individuals taking warfarin and implications for non-vitamin K oral anticoagulants. Am Heart J. 2017;184:150–5.
Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–32.
Hawkins D. Limitations of traditional anticoagulants. Pharmacotherapy. 2004;24:62–5S.
Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34.
Jones DR, Miller GP. Assays and applications in warfarin metabolism: what we know, how we know it and what we need to know. Expert Opin Drug Metab Toxicol. 2011;7:857–74.
Grand’Maison A, Charest AF, Geerts WH. Anticoagulant use in patients with chronic renal impairment. Am J Cardiovasc Drugs. 2005;5:291–305.
Limdi NA, Limdi MA, Cavallari L, et al. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis. 2010;56:823–31.
Limdi NA, Beasley TM, Baird MF, et al. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. 2009;20:912–21.
Patterson SE, Cohn VH. Hepatic drug metabolism in rats with experimental chronic renal failure. BiochemPharmacol. 1984;33:711–6.
Leblond FA, Groux L, Villeneuve JP, Pichette V. Decreased in vivo metabolism of drugs in chronic renal failure. Drug Metab Dispos. 2000;28:1317–20.
Velenosi TJ, Fu AY, Luo S, Wang H, Urquhart BL. Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metab Dispos. 2012;40:1508–14.
Dreisbach AW, Japa S, Gebrekal AB, et al. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther. 2003;73:475–7.
Gong IY, Schwarz UI, Crown N, et al. Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation. PLoS One. 2011;6:e27808.
Ladda MA, Goralski KB. The effects of CKD on cytochrome P450-mediated drug metabolism. Adv Chronic Kidney Dis. 2016;23:67–75.
Brodsky SV, Collins M, Park E, et al. Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:c142–6.
Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–9.
An JN, Ahn SY, Yoon CH, et al. The occurrence of warfarin-related nephropathy and effects on renal and patient outcomes in korean patients. PLoS One. 2013;8:e57661.
Brodsky SV, Satoskar A, Chen J, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54:1121–6.
Ware K, Brodsky P, Satoskar AA, et al. Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J Am Soc Nephrol. 2011;22:1856–62.
Ozcan A, Ware K, Calomeni E, et al. 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol. 2012;35:356–64.
Rizk DV, Warnock DG. Warfarin-related nephropathy: another newly recognized complication of an old drug. Kidney Int. 2011;80:131–3.
Golbin L, Vigneau C, Touchard G, et al. Warfarin-related nephropathy induced by three different vitamin K antagonists: analysis of 13 biopsy-proven cases. Clin Kidney J. 2017;10:381–8.
Gorin Y. The kidney: An organ in the front line of oxidative stress-associated pathologies. Antioxid Redox Signal. 2016;25:639–41.
Krata N, Zagożdżon R, Foroncewicz B, Mucha K. Oxidative stress in kidney diseases: the cause or the consequence? Arch Immunol Ther Exp. 2018;66:211–20.
Crawford A, Fassett RG, Coombes JS, et al. Relationship between antioxidant enzyme genotype and activity and kidney function: a case-control study. Clin Nephrol. 2012;78:135–44.
Sayanthooran S, Magana-Arachchi DN, Gunerathne L, Abeysekera TD, Sooriyapathirana SS. Upregulation of oxidative stress related genes in a chronic kidney disease attributed to specific geographical locations of Sri Lanka. Biomed Res Int. 2016;2016:7546265.
ElGendy AA, Abbas AM. Effects of warfarin and L-carnitine on hemostatic function and oxidative stress in streptozotocin-induced diabetic rats. J Physiol Biochem. 2014;70:535–46.
Onaran I, Sencan S, Demirtaş H, Aydemir B, Ulutin T, Okutan M. Toxic-dose warfarin-induced apoptosis and its enhancement by gamma ionizing radiation in leukemia K562 and HL-60 cells is not mediated by induction of oxidative stress. Naunyn Schmiedeberg’s Arch Pharmacol. 2008;378:471–81.
Nakanishi T, Kuragano T, Nanami M, Nagasawa Y, Hasuike Y. Misdistribution of iron and oxidative stress in chronic kidney disease. Free Radic Biol Med. 2019;133:248–53.
Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB. Association of blood transfusion with acute kidney injury after transcatheter aortic valve replacement: a meta-analysis. World J Nephrol. 2016;5:482–8.
Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. 2010;55:2024–33.
Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–20.
Merle NS, Grunenwald A, Rajaratnam H, et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight. 2018;3:e96910.
Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82–83:969–74.
Gamboa JL, Billings FT 4th, Bojanowski MT, et al. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep. 2016;4:e12780.
Rahman MN, Vukomanovic D, Vlahakis JZ, Szarek WA, Nakatsu K, Jia Z. Structural insights into human heme oxygenase-1 inhibition by potent and selective azole-based compounds. J R Soc Interface. 2013;10:20120697.
Horowitz MP, Greenamyre JT. Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis. 2010;20:S551–68.
Kanakiriya SK, Croatt AJ, Haggard JJ, et al. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol Renal Physiol. 2003;284:F546–54.
Gonzalez-Michaca L, Farrugia G, Croatt AJ, Alam J, Nath KA. Heme: a determinant of life and death in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F370–7.
Kanwar YS. A dynamic interplay between monocyte chemoattractant protein-1 and heme oxygenase-1: implications in renal injury. Kidney Int. 2005;68:896–7.
Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81.
Tsai MT, Chen YY, Chang WJ, Li SY. Warfarin accelerated vascular calcification and worsened cardiac dysfunction in remnant kidney mice. J Chin Med Assoc. 2018;81:324–30.
Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717–22.
Krüger T, Oelenberg S, Kaesler N, et al. Warfarin induces cardiovascular damage in mice. Arterioscler Thromb Vasc Biol. 2013;33:2618–24.
Han KH, O’Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. 2016;5:e002665.
Villines TC, O’Malley PG, Feuerstein IM, Thomas S, Taylor AJ. Does prolonged warfarin exposure potentiate coronary calcification in humans? Results of the warfarin and coronary calcification study. Calcif Tissue Int. 2009;85:494–500.
Andrews J, Psaltis PJ, Bayturan O, et al. Warfarin use is associated with progressive coronary arterial calcification: insights from serial intravascular ultrasound. JACC Cardiovasc Imaging. 2018;11:1315–23.
Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am SocNephrol. 2008;3:1504–10.
Zaragatski E, Grommes J, Schurgers LJ, et al. Vitamin K antagonism aggravates chronic kidney disease-induced neointimal hyperplasia and calcification in arterialized veins: role of vitamin K treatment? Kidney Int. 2016;89:601–11.
Puzantian H, Akers SR, Oldland G, et al. Circulating dephospho-uncarboxylated matrix Gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31:988–94.
Yu WY, Bhutani T, Kornik R, et al. Warfarin-associated nonuremic calciphylaxis. JAMA Dermatol. 2017;153:309–14.
McCabe KM, Booth SL, Fu X, et al. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835–44.
Barcellona D, Vannini ML, Fenu L, Balestrieri C, Marongiu F. Warfarin or acenocoumarol: which is better in the management of oral anticoagulants? Thromb Haemost. 1998;80:899–902.
Suárez-Peñaranda JM, Minasyan A, Sainz-Gaspar L, Sánchez-Aguilar MD. Resolution of acenocoumarol-associated calciphylaxis with drug withdrawal. Australas J Dermatol. 2019;60:e223–6.
Muniesa C, Marcoval J, Moreno A, et al. Coumarin necrosis induced by renal insufficiency. Br J Dermatol. 2004;151:502–4.
Torres-Bondia FI, Parada-Saavedra FJ, Fernández-Armenteros JM, Schoenenberger-Arnaiz JA. Non-uremic calciphylaxis due to acenocoumarol. Farm Hosp. 2017;41:569–70.
Behera SK, Xavier AS, Selvarajan S, Munuswamy H, Haridasan S, Srinivas BH. Acenocoumarol as an alternative anticoagulant in a patient with warfarin-related nephropathy. Br J Clin Pharmacol. 2018;84:1068–71.
Sánchez Soriano RM, Albero Molina MD, Chamorro Fernández CI, et al. Long-term prognostic impact of anticoagulation on patients with atrial fibrillation undergoing hemodialysis. Nefrologia. 2018;38:394–400.
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018;20:1231–42.
Caldeira D, Gonçalves N, Pinto FJ, Costa J, Ferreira JJ. Risk of renal failure with the non-vitamin K antagonist oral anticoagulants: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2015;24:757–64.
Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate. Clin Pharmacokinet. 2010;49:259–68.
Knauf F, Chaknos CM, Berns JS, Perazella MA. Dabigatran and kidney disease: a bad combination. Clin J Am Soc Nephrol. 2013;8:1591–7.
Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (randomized evaluation of long-term anticoagulation therapy) trial analysis. Circulation. 2014;129:961–70.
Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: An analysis from the RE-LY trial. J Am Coll Cardiol. 2015;65:2481–93.
Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131:972–9.
Chan KE, Giugliano RP, Patel MR, et al. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on Dialysis with AF. J Am Coll Cardiol. 2016;67:2888–99.
Chan YH, Yeh YH, See LC, et al. Acute kidney injury in Asians with atrial fibrillation treated with Dabigatran or warfarin. J Am Coll Cardiol. 2016;68:2272–83.
Chan YH, Yeh YH, Hsieh MY, et al. The risk of acute kidney injury in Asians treated with apixaban, rivaroxaban, dabigatran, or warfarin for non-valvular atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. 2018;265:83–9.
Escoli R, Santos P, Andrade S, Carvalho F. Dabigatran-related nephropathy in a patient with undiagnosed IgA nephropathy. Case Rep Nephrol. 2015;2015:298261.
Moeckel GW, Luciano RL, Brewster UC. Warfarin-related nephropathy in a patient with mild IgA nephropathy on dabigatran and aspirin. Clin Kidney J. 2013;6:507–9.
Sharfuddin N, Nourbakhsh M, Box A, Benediktsson H, Muruve DA. Anticoagulant related nephropathy induced by Dabigatran. Case Rep Nephrol. 2018;2018:7381505.
Ryan M, Ware K, Qamri Z, et al. Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant. 2014;29:2228–34.
Gui Y, Loutzenhiser R, Hollenberg MD. Bidirectional regulation of renal hemodynamics by activation of PAR1 and PAR2 in isolated perfused rat kidney. Am J Physiol Renal Physiol. 2003;285:F95–104.
Palygin O, Ilatovskaya DV, Staruschenko A. Protease-activated receptors in kidney disease progression. Am J Physiol Renal Physiol. 2016;311:F1140–4.
Chen B, Soto AG, Coronel LJ, Goss A, van Ryn J, Trejo J. Characterization of thrombin-bound dabigatran effects on protease-activated receptor-1 expression and signaling in vitro. Mol Pharmacol. 2015;88:95–105.
Ware KM, Vance JC, Muni N, et al. Oral warfarin and the thrombin inhibitor dabigatran increase blood pressure in rats: hidden danger of anticoagulants? Am J Hypertens. 2015;28:182–9.
Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol. 2010;70:703–12.
Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–94.
Coleman CI, Kreutz R, Sood N, et al. Effectiveness and safety of rivaroxaban versus warfarin in Nonvalvular atrial fibrillation patients with severe kidney disease or undergoing Hemodialysis. Am J Med. 132:1078–1083https://doi.org/10.1016/j.amjmed.2019.04.013.
Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134:37–47.
Fujino Y, Takahashi C, Mitsumoto K, Uzu T. Rivaroxaban-related acute kidney injury in a patient with IgA vasculitis. BMJ Case Rep. 2019;12:e227756.
Terry CM, He Y, Cheung AK. Rivaroxaban improves patency and decreases inflammation in a mouse model of catheter thrombosis. Thromb Res. 2016;144:106–12.
Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug MetabDispos. 2009;37:74–81.
Chang M, Yu Z, Shenker A, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56:637–45.
Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–30.
Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and safety of Apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1:451–60.
Ishibashi Y, Matsui T, Yamagishi S. Apixaban exerts anti-inflammatory effects in mesangial cells by blocking thrombin/protease-activated receptor-1 system. Thromb Res. 2014;134:1365–7.
Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–53.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–93.
Zhang C, Gu ZC, Ding Z, et al. Decreased risk of renal impairment in atrial fibrillation patients receiving non-vitamin K antagonist oral anticoagulants: a pooled analysis of randomized controlled trials and real-world studies. Thromb Res. 2019;174:16–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scicchitano, P., Tucci, M., Bellino, M.C. et al. The Impairment in Kidney Function in the Oral Anticoagulation Era. A Pathophysiological Insight. Cardiovasc Drugs Ther 35, 505–519 (2021). https://doi.org/10.1007/s10557-020-07004-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07004-x