Abstract
Purpose
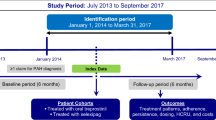
Clinicians may transition patients on parenteral or inhaled prostacyclins to oral treprostinil for ease of use or to avoid adverse effects related to parenteral therapy. However, few data are available to guide these transitions in inpatients. The purpose of this analysis is to describe the inpatient initiation of oral treprostinil at an academic medical system.
Methods
This is a retrospective cohort analysis of patients newly initiated on oral treprostinil at Cleveland Clinic Heath System from 2015 to 2017. Demographic information regarding pulmonary arterial hypertension (PAH) history and previous PAH therapies were recorded. Outcomes evaluated included doses of oral treprostinil utilized, adverse effects related to therapy, and measures of clinical and functional status before and after the initiation of oral treprostinil.
Results
Overall, 29 patients were prescribed oral treprostinil, of which 15 patients were included in the analysis. Common reasons for initiation of oral treprostinil included disease progression (6, 40%) and patient desire (4, 25%). The median duration of transition/initiation of oral treprostinil was 4 days (range, 3–11 days). Median daily dose of oral treprostinil on day 1 of initiation was 2 mg (0.25–4 mg). By day 7, median daily dose was 15 mg (0.75–27.75 mg). Common adverse effects related to therapy were gastrointestinal (7, 47%) and headache (4, 27%). No patients required discontinuation of oral treprostinil due to adverse effects within 90 days of initiation.
Conclusion
Inpatient initiation/transition to oral treprostinil was relatively well tolerated. Future studies should evaluate clinical outcomes surrounding the transitioning to oral treprostinil.

Similar content being viewed by others
References
Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–86.
Kumar P, Thudium E, Laliberte K, Zaccardelli D, Nelsen A. A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet. 2016;55(12):1495–505.
Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132(6):425–34.
Barst RJ, Rubin LJ, Long WA, McGoon M, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301.
Kingman M, Archer-Chicko C, Bartlett M, Beckmann J, Hohsfield R, Lombardi S. Management of prostacyclin side effects in adult patients with pulmonary arterial hypertension. Pulm Circ. 2017;7(3):598–608.
Coons JC, Miller T. Extended-release oral treprostinil in the management of pulmonary arterial hypertension: clinical evidence and experience. Ther Adv Respir Dis. 2018;12:1753466618766490.
Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127(5):624–33.
Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144(3):952–8.
Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142(6):1383–90.
Chin KM, Ruggiero R, Bartolome S, Velez-Martinez M, Darsaklis K, Kingman M, et al. Long-term therapy with oral treprostinil in pulmonary arterial hypertension failed to lead to improvement in important physiologic measures: results from a single center. Pulm Circ. 2015;5(3):513–20.
Tapson VF, Sanchez Diaz CJ, Bohns Meyer GM, Pulido T, Sepulveda P, Wang KY, et al. Treatment with oral treprostinil delays time to clinical worsening in patients with pulmonary arterial hypertension - results from FREEDOM-EV. Journal of Heart and Lung Transplantation. 2019;38(4):S94–5.
Feldman J, Habib N, Radosevich J, Dutt M. Oral treprostinil in the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2017;18(15):1661–7.
Simonneau, G., et al., Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J, 2019. 53(1).
Chakinala MM, Feldman JP, Rischard F, Mathier M, Broderick M, Leedom N, et al. Transition from parenteral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant. 2017;36(2):193–201.
Ackerbauer KA, Tandon R. Transition from subcutaneous or inhaled treprostinil to oral treprostinil at home in patients with pulmonary arterial hypertension: a retrospective case series. J Pharm Pract. 2018;31(2):163–6.
Coons JC, Miller T, Simon MA, Ishizawar DC, Mathier MA. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients transitioned from parenteral or inhaled prostacyclins: case series and treatment protocol. Pulm Circ. 2016;6(1):132–5.
Smith ZR, et al. Transitioning parenteral or inhaled treprostinil to oral treprostinil diolamine: case series and review of the literature. J Pharm Pract. 2018:897190018764585.
Thurber KM, Williams BM, Bates RE, Frantz RP. Transition of intravenous treprostinil to oral therapy in a patient with functional class IV chronic thromboembolic pulmonary hypertension. Pharmacotherapy. 2017;37(8):e76–81.
Gleason JB, et al. The rapid initiation, titration, and transition from intravenous to oral treprostinil in a patient with severe pulmonary arterial hypertension. Case Rep Pulmonol. 2015;2015:498981.
Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–33.
Lachant DJ, Light AN, Mackin ML, Schwartz RG, White RJ. Heart rate expenditure correlates with right ventricular function. Ann Am Thorac Soc. 2020;17(3):372–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Rahaghi reports receiving honoraria for speaking and consulting fees from United Therapeutics. All other authors report no conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Due to the retrospective nature of the analysis, the Institutional Review Board did not require informed consent to be obtained from patients.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hohlfelder, B., Tonelli, A.R., Heresi, G.A. et al. Inpatient Initiation of Oral Treprostinil in an Academic Medical System. Cardiovasc Drugs Ther 34, 547–553 (2020). https://doi.org/10.1007/s10557-020-06992-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-06992-0