Abstract
Purpose
Cardiac fibrosis is characterized by net accumulation of extracellular matrix (ECM) components in the myocardium and facilitates the development of heart failure. C1q/tumor necrosis factor-related protein 15 (CTRP15) is a novel member of the CTRP family, and its gene expression is detected in adult mouse hearts. The present study was performed to determine the effect of CTRP15 on pressure overload-induced fibrotic remodeling.
Methods
Mice were subjected to transverse aortic constriction (TAC) surgery, and adeno-associated virus serotype 9 (AAV9)-carrying mouse CTRP15 gene was injected into mice to achieve CTRP15 overexpression in the myocardium. Adenovirus carrying the gene encoding CTRP15 or small interfering RNA (siRNA) of interest was infected into cultured neonatal mouse ventricular cardiomyocytes (NMVCs) or cardiac fibroblasts (CFs). Gene expression was measured by quantitative real-time PCR, and protein expression and distribution were determined by Western blotting, immunocytochemistry, and immunofluorescence staining.
Results
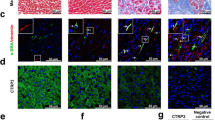
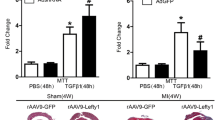
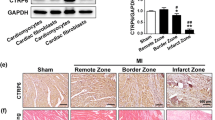
CTRP15 was predominantly produced by cardiac myocytes. CTRP15 expression in the left ventricles was downregulated in mice that underwent TAC. AAV9-mediated CTRP15 overexpression alleviated ventricular remodeling and dysfunction in the pressure-overloaded mice. Treatment of CFs with recombinant CTRP15 or the conditioned medium containing CTRP15 inhibited transforming growth factor (TGF)-β1-induced Smad3 activation and myofibroblast differentiation. CTRP15 increased phosphorylation of insulin receptor (IR), insulin receptor substrate-1 (IRS-1), and Akt. Blockade of IR/IRS-1/Akt pathway reversed the inhibitory effect of CTRP15 on TGF-β1-induced Smad3 activation.
Conclusion
CTRP15 exerts an anti-fibrotic effect on pressure overload-induced cardiac remodeling. The activation of IR/IRS-1/Akt pathway contributes to the anti-fibrotic effect of CTRP15 through targeting Smad3.








Similar content being viewed by others
References
Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79(1):215–62.
Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89(2):265–72.
Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev. 2014;19(2):173–85.
Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307(1–2):159–67.
Espira L, Czubryt MP. Emerging concepts in cardiac matrix biology. Can J Physiol Pharmacol. 2009;87(12):996–1008.
Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-beta. Cardiovasc Ther. 2012;30(1):e30–40.
Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15.
Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74(2):207–12.
Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–7.
Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120(1):254–65.
Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10(1):15–26.
Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A. 2005;102(2):437–42.
Shi Q, Liu X, Bai Y, Cui C, Li J, Li Y, et al. In vitro effects of pirfenidone on cardiac fibroblasts: proliferation, myofibroblast differentiation, migration and cytokine secretion. PLoS One. 2011;6(11):e28134.
Stempien-Otero A, Kim DH, Davis J. Molecular networks underlying myofibroblast fate and fibrosis. J Mol Cell Cardiol. 2016;97:153–61.
Schaffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab. 2012;23(4):194–204.
Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287(15):11968–80.
Seldin MM, Lei X, Tan SY, Stanson KP, Wei Z, Wong GW. Skeletal muscle-derived myonectin activates the mammalian target of rapamycin (mTOR) pathway to suppress autophagy in liver. J Biol Chem. 2013;288(50):36073–82.
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84.
Jiang X, Gao M, Chen Y, Liu J, Qi S, Ma J, et al. EPO-dependent induction of erythroferrone drives hepcidin suppression and systematic iron absorption under phenylhydrazine-induced hemolytic anemia. Blood Cells Mol Dis. 2016;58:45–51.
Latour C, Wlodarczyk MF, Jung G, Gineste A, Blanchard N, Ganz T, et al. Erythroferrone contributes to hepcidin repression in a mouse model of malarial anemia. Haematologica. 2017;102(1):60–8.
Otaka N, Shibata R, Ohashi K, Uemura Y, Kambara T, Enomoto T, et al. Myonectin is an exercise-induced Myokine that protects the heart from ischemia-reperfusion injury. Circ Res. 2018;123(12):1326–38.
Yuan Y, Lau WB, Su H, Sun Y, Yi W, Du Y, et al. C1q-TNF-related protein-9, a novel cardioprotective cardiokine, requires proteolytic cleavage to generate a biologically active globular domain isoform. Am J Physiol Endocrinol Metab. 2015;308(10):E891–8.
Zhang CL, Zhao Q, Liang H, Qiao X, Wang JY, Wu D, et al. Cartilage intermediate layer protein-1 alleviates pressure overload-induced cardiac fibrosis via interfering TGF-beta1 signaling. J Mol Cell Cardiol. 2018;116:135–44.
Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–83.
Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279(1):H429–36.
Chen R, Feng Y, Wu J, Song Y, Li H, Shen Q, et al. Metformin attenuates angiotensin II-induced TGFbeta1 expression by targeting hepatocyte nuclear factor-4-alpha. Br J Pharmacol. 2018;175(8):1217–29.
Ehler E, Moore-Morris T, Lange S. Isolation and culture of neonatal mouse cardiomyocytes. J Vis Exp. 2013;79:e50154.
Pare B, Deschenes LT, Pouliot R, Dupre N, Gros-Louis F. An optimized approach to recover secreted proteins from fibroblast conditioned-media for sSecretomic analysis. Front Cell Neurosci. 2016;10:70.
Kumar S, Seqqat R, Chigurupati S, Kumar R, Baker KM, Young D, et al. Inhibition of nuclear factor kappaB regresses cardiac hypertrophy by modulating the expression of extracellular matrix and adhesion molecules. Free Radic Biol Med. 2011;50(1):206–15.
Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–74.
Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat Cell Biol. 2004;6(4):358–65.
Yuasa D, Ohashi K, Shibata R, Mizutani N, Kataoka Y, Kambara T, et al. C1q/TNF-related protein-1 functions to protect against acute ischemic injury in the heart. FASEB J. 2016;30(3):1065–75.
Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, et al. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J Mol Med (Berl). 2015;93(12):1311–25.
Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng H, et al. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol. 2015;110(4):35.
Kambara T, Shibata R, Ohashi K, Matsuo K, Hiramatsu-Ito M, Enomoto T, et al. C1q/tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-dependent mechanism. Mol Cell Biol. 2015;35(12):2173–85.
Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, et al. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase a activation. Circulation. 2013;128(11 Suppl 1):S113–20.
Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, et al. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47.
Wei WY, Ma ZG, Zhang N, Xu SC, Yuan YP, Zeng XF, et al. Overexpression of CTRP3 protects against sepsis-induced myocardial dysfunction in mice. Mol Cell Endocrinol. 2018;476:27–36.
Ma ZG, Yuan YP, Xu SC, Wei WY, Xu CR, Zhang X, et al. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60(6):1126–37.
Bai S, Cheng L, Yang Y, Fan C, Zhao D, Qin Z, et al. C1q/TNF-related protein 9 protects diabetic rat heart against ischemia reperfusion injury: role of endoplasmic reticulum stress. Oxidative Med Cell Longev. 2016;2016:1902025.
Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285(5):H1871–81.
Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–55.
Pichler M, Rainer PP, Schauer S, Hoefler G. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol. 2012;59(11):1008–16.
Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, et al. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287(23):18965–73.
Han CY, Koo JH, Kim SH, Gardenghi S, Rivella S, Strnad P, et al. Hepcidin inhibits Smad3 phosphorylation in hepatic stellate cells by impeding ferroportin-mediated regulation of Akt. Nat Commun. 2016;7:13817.
Acknowledgments
Authors acknowledge Dr. Yao Song (Peking University Third Hospital, Beijing, China) for technical support in echocardiographic examination. We also acknowledge Dr. Zhen-Zhen Chen (Peking University School of Basic Medical Sciences, Beijing, China) for helping to inject AAV9 in animal experiments.
Funding
This work is supported by grants from the National Natural Science Foundation of China (81770225 and 81370192).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were conducted with the approval of the Ethics Committee of Animal Research, Peking University Health Science Center, and the investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qian Zhao and Cheng-Lin Zhang contribute equally to this work.
Electronic supplementary material
ESM 1
(DOCX 191 kb)
Rights and permissions
About this article
Cite this article
Zhao, Q., Zhang, CL., Xiang, RL. et al. CTRP15 derived from cardiac myocytes attenuates TGFβ1-induced fibrotic response in cardiac fibroblasts. Cardiovasc Drugs Ther 34, 591–604 (2020). https://doi.org/10.1007/s10557-020-06970-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-06970-6