Abstract
Objective
Permanent polymer drug-eluting stents (DES) are associated with a higher risk of late and very late stent thrombosis (ST); biodegradable polymer drug-eluting stents (BP-DES) were designed to reduce these risks. However, their benefits are not completely clear.
Method
We undertook a meta-analysis of randomized studies identified in systematic searches of MEDLINE, EMBASE, and the Cochrane Database. Eligible studies were those that compared BP-DES with second-generation permanent polymer DES in patients undergoing percutaneous coronary intervention.
Results
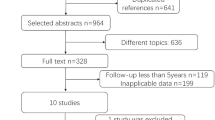
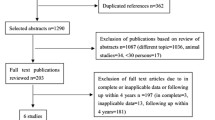
Five studies (8,740 patients) with a mean follow-up of 19.2 months were included. Overall, BP-DES were associated with a broadly equivalent risk of definite and probable ST (odds ratio [OR], 1.07; 95 % confidence interval [CI], 0.67 to 1.71; P = 0.76; I 2 = 5.0 %), target vessel revascularization (OR, 1.04; 95 % CI, 0.87 to 1.24; P = 0.68; I 2 = 38.0 %), all-cause mortality (OR, 1.10; 95 % CI, 0.87 to 1.41; P = 0.42; I 2 = 0.0 %), and major adverse cardiac events (OR, 1.03; 95 % CI, 0.88 to 1.20; P = 0.74; I 2 = 0.0 %) when compared with second-generation DES. However, BP-DES significantly decreased in-stent late luminal loss (standard mean difference [SMD], −0.01; 95 % CI, −0.12 to 0.11; P = 0.93; I 2 = 0.0 %) and in-segment late luminal loss (SMD, −0.06; 95 % CI, −0.17 to 0.05; P = 0.27; I 2 = 0.0 %) compared with second-generation DES.
Conclusions
Compared with second-generation permanent polymer DES, biodegradable stents appear to have equivalent short- to medium-term clinical benefits, and it remains unclear whether they reduce the incidence of very late ST.






Similar content being viewed by others
References
Kastrati A, Dibra A, Spaulding C, Laarman GJ, Menichelli M, Valgimigli M, et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007;28:2706–13.
Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–402.
Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Valgimigli M, et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with st-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496–504.
Garg S, Bourantas C, Serruys PW. New concepts in the design of drug-eluting coronary stents. Nat Rev Cardiol. 2013;10:248–60.
Sammel AM, Chen D, Jepson N. New generation coronary stent technology–is the future biodegradable? Heart Lung Circ. 2013;22:495–506.
Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, Nakagawa Y, et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181–90.
Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (compare ii): a randomised, controlled, non-inferiority trial. Lancet. 2013;381:651–60.
Gao RL, Xu B, Lansky AJ, Vázquez N, Valdés M, Voudris V, et al. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the TARGET I trial. EuroIntervention. 2013;9:75–83.
Giulio GS, David RH. Drug-eluting coronary artery stent. N Engl J Med. 2013;368:254–65.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–6.
Byrne RA, Kastrati A, Massberg S, Wieczorek A, Laugwitz KL, Hadamitzky M, et al. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2011;58:1325–31.
Meredith IT, Verheye S, Dubois CL, Dens J, Fajadet J, Carrié D, et al. Primary endpoint results of the evolve trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59:1362–70.
Rodriguez-Granillo A, Rubilar B, Rodriguez-Granillo G, Rodriguez AE. Advantages and disadvantages of biodegradable platforms in drug eluting stents. World J Cardiol. 2011;3:84–92.
Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the leaders (limus eluted from a durable versus erodable stent coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777–89.
Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the isar-test 3, isar-test 4, and leaders randomized trials. Eur Heart J. 2012;33:1214–22.
Ormiston JA, Webster MW. Stent thrombosis: has the firestorm been extinguished? Lancet. 2012;379:1368–9.
Pendyala LK, Matsumoto D, Shinke T, Iwasaki T, Sugimoto R, Hou D, et al. Nobori stent shows less vascular inflammation and early recovery of endothelial function compared with cypher stent. JACC Cardiovasc Interv. 2012;5:436–44.
Tada T, Byrne RA, Schuster T, Cuni R, Kitabata H, Tiroch K, et al. Early vascular healing with rapid breakdown biodegradable polymer sirolimus-eluting versus durable polymer everolimus- eluting stents assessed by optical coherence tomography. Cardiovasc Revasc Med. 2013;14:84–9.
De Luca G, Suryapranata H, Stone GW, Antoniucci D, Biondi-Zoccai G, Kastrati A, et al. Coronary stenting versus balloon angioplasty for acute myocardial infarction: a meta- regression analysis of randomized trials. Int J Cardiol. 2008;126:37–44.
Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, et al. Incidence and predictors of restenosis after coronary stenting in 10004 patients with surveillance angiography. Heart. 2013. doi:10.1136/heartjnl-2013 -304933.
De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, et al. Drug-eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch Intern Med. 2012;172:611–21.
Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, Hannan EL, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013. doi:10.1136/bmj.f6625.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Dong, P., Li, L. et al. Biodegradable Polymer Drug-Eluting Stents Versus Second-Generation Drug-Eluting Stents for Patients With Coronary Artery Disease: An Update Meta-Analysis. Cardiovasc Drugs Ther 28, 379–385 (2014). https://doi.org/10.1007/s10557-014-6528-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-014-6528-7