Abstract
Purpose
Volume overload is a common complication associated with heart failure (HF) and is recommended to be treated with loop or thiazide diuretics. However, use of diuretics can cause serum electrolyte imbalances and diuretic resistance. Tolvaptan, a selective, oral, non-peptide vasopressin V2-receptor antagonist, offers a new option for treating volume overload in HF patients. The aim of this study was to investigate the efficacy and safety of tolvaptan in Japanese HF patients with volume overload.
Methods
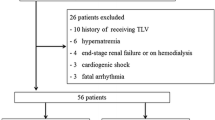
Fifty-one HF patients with volume overload, despite using conventional diuretics, were treated with 15 mg/day tolvaptan for 7 days. If the response was insufficient at Day 7, tolvaptan was continued for a further 7 days at either 15 mg/day or 30 mg/day. Outcomes included changes in body weight, symptoms and safety parameters.
Results
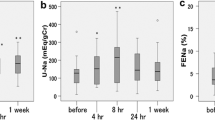
Thirty-six patients discontinued treatment within 7 days, therefore 15 patients entered the second phase of treatment. In two patients, tolvaptan was increased to 30 mg/day after 7 days. Body weight was reduced on Day 7 (−1.95 ± 1.98 kg; n = 41) and Day 14 (−2.35 ± 1.44 kg; n = 11, 15 mg/day). Symptoms of volume overload, including lower limb edema, pulmonary congestion, jugular venous distention and hepatomegaly, were improved by tolvaptan treatment for 7 or 14 days. Neither tolvaptan increased the incidence of severe or serious adverse events when administered for 7–14 days.
Conclusions
This study confirms the efficacy and safety of 15 mg/day tolvaptan for 7–14 days in Japanese HF patients with volume overload despite conventional diuretics.







Similar content being viewed by others
References
Japanese Circulation Society. Guidelines for the diagnosis and treatment of cardiovascular disease (Joint Study Group Report 2009): guidelines for treatment of chronic heart failure (revised 2010).http://www.j-circ.or.jp/guideline/pdf/JCS2010_matsuzaki_h.pdf . Accessed 13 April 2011.
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804.
Raftery EB. Haemodynamic effects of diuretics in heart failure. Br Heart J. 1994;72(suppl):S44–7.
Anand I, Florea VG. Diuretics in chronic heart failure—benefits and hazards. Eur Heart J Suppl. 2001;3(Suppl G):G8–G18.
Gupta S, Neyses L. Diuretic usage in heart failure: a continuing conundrum in 2005. Eur Heart J. 2005;26:644–9.
Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235.
Yamamura Y, Nakamura S, Itoh S, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–7.
Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107:2690–6.
Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273–8.
Okita K, Sakaida I, Okada M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose–response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–87.
Matsuzaki M, Masatsugu H, Izumi T, Asanoi H, Tsutamoto T, Tolvaptan Investigators. Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Cardiovasc Drugs Ther. 2011; e-pub ahead of print.
Matsuzaki M, Fukunami M, Hori M, Izumi T, Tolvaptan Investigators. Efficacy and safety of tolvaptan in heart failure patients with excessive fluid retention following treatment with other diuretics: a phase iii, randomized, double-blinded and placebo-controlled study (The QUEST study). Cardiovasc Drugs Ther. 2011; e-pub ahead of print.
Disclosures
Editorial support for this manuscript was provided by Elsevier Japan K.K. This support was funded by Otsuka Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
The present study was conducted at the following institutions in Japan: National Hospital Organization Hakodate National Hospital, Hokkaido Prefectural Welfare Federation of Agricultural Cooperatives Sapporo Kosei General Hospital, National Hospital Organization Nishi Sapporo National Hospital, National Hospital Organization Tokyo Medical Center, Tokyo Medical University Hospital, Nihon University Itabashi Hospital, National Hospital Organization Takasaki General Medical Center, National Hospital Organization Saitama National Hospital, University of Yamanashi Hospital, Nagano Prefectural Federation of Agricultural Cooperatives for Health and Welfare Saku Central Hospital, Seirei Hamamatsu General Hospital, Mie University Hospital, Mie Prefectural General Medical Center, National Cerebral and Cardiovascular Center, Osaka Police Hospital, Osaka City General Hospital, Kansai Rosai Hospital, Nara Medical University Hospital, Hospital of Hyogo College of Medicine, Hyogo Prefectural Amagasaki Hospital, Tokushima University Hospital, National Hospital Organization Ureshino Medical Center, and Saiseikai Kumamoto Hospital.
Rights and permissions
About this article
Cite this article
Fukunami, M., Matsuzaki, M., Hori, M. et al. Efficacy and Safety of Tolvaptan in Heart Failure Patients with Sustained Volume Overload despite the Use of Conventional Diuretics: A Phase III Open-Label Study. Cardiovasc Drugs Ther 25 (Suppl 1), 47–56 (2011). https://doi.org/10.1007/s10557-011-6348-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6348-y