Abstract
Purpose
Tolvaptan may reduce the signs of volume overload in heart failure (HF) patients who experience volume overload despite using conventional diuretics. In this study, we evaluated the dose-response effects of tolvaptan on weight loss, urine volume and electrolyte excretion in furosemide-treated Japanese HF patients exhibiting volume overload.
Methods
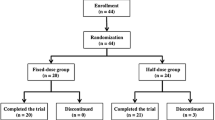
In the study, 117 HF patients with volume overload on stable doses of furosemide (≥40 mg/day) were treated with tolvaptan (15, 30 or 45 mg) or placebo once-daily for 7 days.
Results
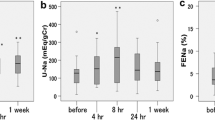
The decrease in body weight from baseline to the day after the final dose with 15, 30 or 45 mg tolvaptan (–1.62 ± 1.55, –1.35 ± 1.54 and –1.85 ± 1.10 kg, respectively), was significantly greater compared with that in the placebo group (–0.53 ± 0.96 kg) (p < 0.05). However, the decrease in body weight with tolvaptan was not significantly dose-dependent. Signs of volume overload improved at all doses of tolvaptan. Tolvaptan elicited a dose-dependent increase in urine volume and a decrease in urine osmolality, but did not affect urinary sodium or potassium excretion. Adverse reactions associated with diuresis were most frequently observed at the higher doses of tolvaptan.
Conclusions
Once-daily tolvaptan (15, 30 or 45 mg) was effective and tolerable as an add-on treatment to furosemide therapy in Japanese HF patients with volume overload.



Similar content being viewed by others
References
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identified hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804.
Gupta S, Neyses L. Diuretic usage in heart failure: a continuing conundrum in 2005. Eur Heart J. 2005;26:644–9.
Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohormonal axis. Ann Intern Med. 1985;103:1–6.
Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107:2690–6.
Japanese Circulation Society. Guidelines for the Treatment of Chronic Heart Failure, 2010. Available at: http://www.j-circ.or.jp/guideline/pdf/JCS2010_matsuzaki_h.pdf. Accessed 13 April 2011.
Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235.
Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–87.
Schrier R. The patient with hyponatremia or hypernatremia. In: Schrier R, editor. Manual of nephrology. 4th ed. Boston: Little, Brown & Co; 1995. p. 20–36.
Zeidel M. Special diuretics. In: Seldin D, Giebisch G, editors. Diuretic agents: clinical physiology and pharmacology. San Diego: Academic Press Inc; 1997. p. 113–34.
Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–43.
Ghali JK, Hamad B, Yasothan U, Kirkpatrick P. Tolvaptan. Nat Rev Drug Discov. 2009;8:611–12.
Hunt SA, Abraham WT, Chin MH, et al. 2009 focused updated incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90.
Muller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793–801.
Chen HH, Schrier RW. Pathophysiology of volume overload in acute heart failure syndromes. Am J Med. 2006;119:S11–6.
Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist—pharmacology and clinical trials. Cardiovasc Drug Rev. 2007;25:1–13.
Costello-Boerringter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273–8.
Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31.
Disclosures
None of the authors have any conflicts of interest associated with this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix: Participating investigators and institutions
Appendix: Participating investigators and institutions
The following investigators and institutions participated in this trial: Akihiro Azuma, University Hospital, Kyoto Prefectural University of Medicine, Kyoto; Yoshinori Doi, Kochi Medical School Hospital, Nankoku, Kochi; Kenji Fujii, Sakurabashi Watanabe Hospital, Osaka; Kazuteru Fujimoto, National Hospital Organization Kumamoto Medical Center, Kumamoto; Jun Fuse, National Hospital Organization Tokyo Medical Center, Meguro, Tokyo; Hiroyuki Hanada, Hirosaki University School of Medicine & Hospital, Hirosaki, Aomori; Michiaki Hiroe, National Center for Global Health and Medicine, Shinjuku, Tokyo; Hitoshi Hishida, Fujita Health University, Toyoake, Aichi; Hiroaki Hosokawa, National Hospital Organization Toyohashi Medical Center, Toyohashi, Aichi; Takayuki Inomata, Kitasato University Hospital, Sagamihara, Kanagawa; Ryoji Ishiki, Toyota Memorial Hospital, Toyota, Aichi; Hideo Izawa, Nagoya University Hospital, Nagoya, Aichi; Toshio Kasai, National Hospital Organization Nagano National Hospital, Nagano; Shu Kasama, Cardiovascular Hospital of Central Japan, Seta-gun, Gunma; Shun-ichi Kobayashi, Kanagawa Cardiovascular and Respiratory Center, Yokohama, Kanagawa; Masahiko Koda, Gifu Prefectural General Medical Center, Gifu; Sunao Kojima, Kumamoto University Hospital, Kumamoto; Kengo Matsumoto, National Hospital Organization Kure Medical Center, Kure, Hiroshima; Takahiro Matsumoto, National Hospital Organization Kyushu Medical Center, Fukuoka; Tatsuru Matsuoka, National Hospital Organization Kagoshima Medical Center, Kagoshima; Shinji Miki, Mitsubishi Kyoto Hospital, Kyoto; Kazuaki Mitsudo, Kurashiki Central Hospital, Kurashiki, Okayama; Toshiro Miura, Yamaguchi University Hospital, Ube, Yamaguchi; Shigeyuki Nishimura, Saitama Medical University Hospital, Iruma-gun, Saitama; Ryuji Nohara, Kitano Hospital, Osaka; Akira Nozaki, Kanto Central Hospital of the Mutual Aid Association of Public School Teachers, Setagaya, Tokyo; Hiroshi Ogawa, Tokuyama Central Hospital, Shunan, Yamaguchi; Yoshihiko Sakai, Dokkyo Medical University Koshigaya Hospital, Koshigoe, Saitama; Mamoru Sato, Iwate Medical University Hospital, Morioka, Iwate; Ikuo Segawa, Iwate Medical University Hospital, Morioka, Iwate; Yoshihiko Seino, Nippon Medical School Hospital, Bunkyo, Tokyo; Junya Shite, Kobe University Hospital, Kobe, Hyogo; Jun-ichi Suzuki, Tokyo Medical and Dental University, Bunkyo, Tokyo; Masahiro Suzuki, National Hospital Organization Saitama National Hospital, Wako, Saitama; Shuichi Taguchi, National Hospital Organization Mito Medical Center, Ibaraki; Yoshifumi Takada, Tokyo Medical University Hospital, Shinjuku, Tokyo; Keiji Tanaka, Nippon Medical School Hospital, Bunkyo, Tokyo; Masaru Tanaka, Osaka Red Cross Hospital, Osaka; Jun Tanouchi, Osaka Rosai Hospital, Sakai, Osaka; Chuwa Tei, Kagoshima University Medical And Dental Hospital, Kagoshima; Hitoshi Toda, Kagoshima City Hospital, Kagoshima; Kazufumi Tsuchihashi, Sapporo Medical University Hospital, Sapporo, Hokkaido; Hitoshi Yasumoto, Kokura Memorial Hospital, Kitakyushu, Fukuoka; Yoshio Yasumura, National Hospital Organization Osaka National Hospital, Osaka; Hiroyuki Yokoyama, National Hospital Organization Shizuoka Medical Center, Suntou gun, Shizuoka.
Rights and permissions
About this article
Cite this article
Matsuzaki, M., Hori, M., Izumi, T. et al. Effects of Tolvaptan on Volume Overload in Japanese Patients with Heart Failure: Results of a Phase II, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Study. Cardiovasc Drugs Ther 25 (Suppl 1), 19–31 (2011). https://doi.org/10.1007/s10557-011-6303-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6303-y