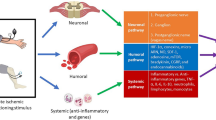
Abstract
Ischemic heart failure is one of the leading causes of death in the western countries and it is a critical issue to overcome ischemic heart diseases for the human health care worldwide. There are several aspects of ischemic heart failure that we need to seriously consider for the conquest of cardiovascular death. First of all, we need to know either causes or pathophysiology of the onset of coronary artery disease, the ischemia/reperfusion injury and post-infarction cardiac remodeling. Secondly, we need to find the potential seeds for the molecular, pharmacological, biomedical or engineering treatment to prevent or attenuate ischemic heart diseases. Thirdly, we need to accelerate translational research and to create the network of clinical trials to grow the novel seeds to the fruitful big trees. Finally, we need to justify these strategies to overcome the ischemic heart diseases and to contribute the world welfare systems after we propose the novel therapy for the prevention and attenuation of ischemic heart diseases. The most strong and essential hypotheses to attenuate the cardiovascular injury in ischemic heart disease for last three decades are ischemic preconditioning/postconditioning. Many investigators have involved in the clarification of the characteristics of ischemic preconditioning/postconditioning and their cellular mechanisms, and the clinical applications of their basic results. Here, 8 potential basic and clinical researchers includeing us discuss these issues that they have devotedly studies for many years.

Similar content being viewed by others
References
Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–918.
Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–67.
Mubagwa K, Mullane K. Flameng Role of adenosine in the heart and circulation. Cardiovasc Res. 1996;32:797–813.
Kitakaze M, Minamino T, Node K, et al. Adenosine and cardioprotection in the diseased heart. Jpn Circ J. 1999;63:231–43.
Chulz R, Cohen MV, Behrends M, Downey JM, Heusch G. Signal transduction of ischemic preconditioning. Cardiovasc Res. 2001;52:181–98.
Kitakaze M. It is the time to ask what adenosine can do for cardioprotection in ischemic heart disease. Internal Medicine. 1999;38:305–6.
Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987;60:153–68.
Mauser M, Hoffmeister HM, Nienaber C, Schaper W. Influence of ribose, adenosine, and “AICAR” on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circ Res. 1985;56:220–30.
Pike MM, Luo CS, Clark D, et al. NMR measurements of Na+ and cellular energy in the ischemic rat heart: role of Na+/H+ exchange. Am J Physiol. 1993;265:H2017–26.
Marban E, Kitakaze M, Kusuoka H, Porterfield JP, Yue DT, Chacko VP. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts containing the Ca2+-indicator 5,5′-F2-BAPTA. Proceeding National Academy Science (U.S.A) 1987;84:6005-9.
Sato H, Hori M, Kitakaze M, et al. Reperfusion after brief ischemia disrupts the microtuble structure in the canine hearts. Circ Res. 1993;72:361–75.
Kitakaze M, Weisman HF, Marban E. Contractile dysfunction and ATP depletion following transient calcium overload in perfused ferret hearts. Circulation. 1988;77:685–95.
Kitakaze M, Weisfeldt ML, Marban E. Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts. J Clin Invest. 1988;82:920–7.
Kitakaze M, Takashima S, Minamino T, et al. Transient acidosis during early reperfusion following myocardial ischemia limits infarct size in the dogs. Am J Physiol. 1997;272:H2071–8.
Taga R, Okabe E. Hydroxyl radical participation in the in vitro effects of gram-negative endotoxin on cardiac sarcolemmal Na, K-ATPase activity. Jpn J Pharmacol. 1991;55:339–49.
Kitakaze M, Hori M, Takashima S, et al. Superoxide dismutase enhances ischemia-induced reactive hyperemic flow and adenosine release in dogs: a role of 5′-nucleotidase activity. Circ Res. 1992;71:558–66.
Gross GJ, Farber NE, Hardman HF, Warltier DC. Beneficial actions of superoxide dismutase and catalase in stunned myocardium of dogs. Am J Physiol. 1986;250:H372–7.
Sekili S, McCay PB, Li XY, et al. Direct evidence that the hydroxyl radical plays a pathogenetic role in myocardial “stunning” in the conscious dog and demonstration that stunning can be markedly attenuated without subsequent adverse effects. Circ Res. 1993;73:705–23.
Flaherty JT, Pitt B, Gruber JW, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–91.
Schömig A, Dart AM, Dietz R, Mayer E, Kübler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part A: locally mediated release. Circ Res. 1984;55:689–701.
Kitakaze M, Hori M, Tamai J, et al. α1-Adrenoceptor activity regulates release of adenosine from the ischemic myocardium in dogs. Circ Res. 1987;60:631–9.
Kitakaze M, Hori M, Gotoh K, et al. Beneficial effects of α2-activity on ischemic myocardium during coronary hypoperfusion in dogs. Circ Res. 1989;65:1632–45.
Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res. 1988;62:286–98.
Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1987;54:1496–508.
Ito H, Maruyama A, Iwakura K, Takiuchi S, et al. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 1996;223–8.
Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–85.
Watanabe T, Suzuki N, Shimamoto N, Fujino M, Imada A. Contribution of endogenous endothelin to the extension of myocardial infarct size in rats. Circ Res. 1991;69:370–7.
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–8.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36.
Kuzuya T, Hoshida S, Yamashita N, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9.
Marbar MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress associated with resistance to myocardial infarction. Circulation. 1993;83:13–25.
Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther 2010;24: this issue.
Mura T, Tanno M. Mitochondria and GSK-3β in cardioprotection against ischemia/reperfusion injury. Cardiovasc Drugs Ther 2010;24: this issue.
Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP). Where are we now? Cardiovasc Drugs Ther 2010;24: this issue.
Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86.
Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88.
Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85.
Sun HY, Wang NP, Kerendi F, et al. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288:H1900–8.
Zhao Z-Q. Postconditioning in reperfusion: a status report. Cardiovasc Drugs Ther 2010;24:this issue.
Ishihara M, Sato H, Tateishi H, et al. Implications of prodromal angina pectoris in anterior wall acute myocardial infarction: acute angiographic findings and long-term prognosis. J Am Coll Cardiol. 1997;30:970–5.
Ross AM, Gibbons RJ, Stone GW, Kloner RA. Alexander RW; AMISTAD-II Investigators. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol. 2005;45:1775–80.
Kitakaze M, Asakura M, Kim J, et al. On behalf of the J-WIND investigators. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–93.
Asakura M. Cardioprotection in the clinical setting—Lesson from J-WIND. Cardiovasc Drugs Ther 2010;24: this issue.
Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–81.
Ivanes F, Mewton N, Rioufol G, Piot C, Elbaz M, Revel D, et al. Cardioprotection in the clinical setting. Cardiovasc Drugs Ther 2010;24: this issue.
Acknowledgements
This work is supported by Grants-in-aid from the Ministry of Health, Labor, and Welfare, Japan and Grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan and Grants from the Japan Cardiovascular Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitakaze, M. How to Mediate Cardioprotection in Ischemic Hearts—Accumulated Evidence of Basic Research Should Translate to Clinical Medicine. Cardiovasc Drugs Ther 24, 217–223 (2010). https://doi.org/10.1007/s10557-010-6248-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-010-6248-6